The Tactics and Tools to Manage Pediatric Heart Failure
August 1, 2020
Related Articles
-
Echocardiographic Estimation of Left Atrial Pressure in Atrial Fibrillation Patients
-
Philadelphia Jury Awards $6.8M After Hospital Fails to Find Stomach Perforation
-
Pennsylvania Court Affirms $8 Million Verdict for Failure To Repair Uterine Artery
-
Older Physicians May Need Attention to Ensure Patient Safety
-
Documentation Huddles Improve Quality and Safety
AUTHORS
Nick Pokrajac, MD, Clinical Assistant Professor, Department of Emergency Medicine, Stanford School of Medicine, Stanford, CA
N. Ewen Wang, MD, Professor of Emergency Medicine, Stanford School of Medicine, Stanford, CA
PEER REVIEWER
Beth Bubolz, MD, Assistant Professor of Pediatrics, Ohio State College of Medicine; Attending Physician in Pediatric Emergency Medicine, Nationwide Children's Hospital, Columbus, OH; Board-certified in Pediatric Cardiology/Electrophysiology
EXECUTIVE SUMMARY
• Nearly half of emergency department (ED) visits by pediatric patients with heart failure are by those younger than 1 year of age, with no major differences in prevalence between males and females. Additionally, the frequency of ventricular assist device (VAD) implantation in children with severe heart failure is increasing, and select centers have started to transition VAD-supported children to outpatient care.
• Forty-nine percent of pediatric patients with heart failure were diagnosed incorrectly at their initial presentation to the ED. Common incorrect diagnoses included pneumonia, viral syndrome, gastroenteritis, asthma, and constipation.
• Physical exam findings for heart failure include the presence of a cardiac murmur or gallop; signs of left ventricular (LV) failure or aortic outflow obstruction (unexplained tachycardia, tachypnea, signs of respiratory distress, and/or rales on auscultation); signs of hypoperfusion (pallor, delayed capillary refill time, diminished pulses, and/or cool extremities); and signs of right ventricular (RV) failure (elevated jugular venous pressure and/or hepatomegaly). No single feature obtained in the history or physical exam is diagnostic, but a combination of findings is worrisome and should be explored further.Implementing a clinical pathway for pediatric ED asthma management leads to improved adherence to evidence-based practices and fewer hospital admissions.
• Structural heart disease is the most common etiology for pediatric heart failure and includes the spectrum of congestive heart disease syndromes (e.g., tetralogy of Fallot, single ventricle, systemic right ventricle, valvular defects, septal wall defects, and coronary artery anomalies). Heart failure in structural heart disease is the end result of altered pressures within the chambers of the heart due to anatomic abnormalities, abnormal systemic-pulmonary connections (shunting), or as a result of ischemia, as with coronary artery anomalies.
• Clinical signs of cardiogenic shock in pediatric patients include altered mental status, abnormal skin perfusion (cold shock, delayed capillary refill time, cool extremities, and/or mottling), diminished quality of pulses, low blood pressure, and low urine output (< 1 mL/kg/hour). Tachycardia also is an important finding, but it is not reliable in children because of the multitude of factors affecting heart rate, such as agitation and fever.
• Electrocardiogram and chest radiograph are useful screening tools for heart failure. Brain natriuretic peptide has a reported sensitivity between 71% and 93% and specificity between 63% and 95% to detect new or worsening structural and functional cardiac disease in children.
• Providers should consider hemodynamic optimization prior to invasive airway management if intubation is not required immediately. If intubation is necessary, induction agents with minimal cardiac depressant effects, such as ketamine, are preferable.
• In severe asthma exacerbations not responsive to initial bronchodilator or steroid therapy and where alternative diagnoses have been discussed, the next tier of therapy is indicated. This includes consideration of terbutaline, epinephrine, and intravenous magnesium.
• VAD placement is indicated for heart failure patients refractory to medical therapy as a bridge to transplant or recovery, or to palliate severe symptoms.
• Among patients listed in a national registry, 70% with pulsatile flow devices and 55% with continuous flow devices had at least one adverse event during the life of the device.
• Alarms indicate impending or existing failure of external components (e.g., batteries and/or controller) or internal components (pump), or episodic dysfunction, such as during a suction event.
• Patients with a continuous flow device lack pulses at baseline, but when they are in circulatory arrest, they will be unresponsive, lack respiratory effort, and have signs of poor perfusion (e.g., delayed or absent capillary refill and/or cool extremities).
Fortunately, pediatric heart failure is a rare occurrence, but early diagnosis, aggressive management, and timely transfer to a facility capable of advanced cardiac support are essential to optimize the outcome of each child. The authors review the early recognition of a child in heart failure and also discuss an approach to troubleshooting and recognizing complications associated with a ventricular assist device.
— Ann M. Dietrich, MD, FAAP, FACEP, Editor
Introduction
Pediatric patients with heart disease, or any structural or functional abnormality of the heart, represent a challenge for emergency department (ED) providers. Because heart disease is rare in the pediatric population, these patients present to the ED far less commonly than adults. Some clinical features of heart disease, such as vomiting, are nonspecific and are more likely to be due to a more frequent, benign condition (e.g., gastroenteritis).1-3
Additionally, due to advancing technology, emergency physicians must be familiar with mechanical circulatory support devices in advanced pediatric heart failure patients. While pediatric heart disease includes a number of clinically important disease entities, such as congenital heart disease and dysrhythmias, this review will focus on pediatric patients with heart failure, including those with ventricular assist devices (VADs). Moreover, evaluation and management of patients with automated implantable cardioverter defibrillators, which often are used in the heart failure population, are beyond the scope of this article and will not be discussed. The first part of this review will provide an epidemiologic overview of pediatric heart failure, while the second part will examine both the history and physical exam findings of pediatric patients in heart failure — and how they differ from adults. Third, the underlying causes, diagnostic evaluation, and recommended management of pediatric patients in heart failure will be discussed. Finally, this article will provide a tutorial for evaluation and management of a pediatric VAD patient.
Epidemiology
Numerous conditions are responsible for pediatric heart failure. Some conditions that result in acute heart failure, such as myocarditis, also lack uniform diagnostic criteria.4,5 As a result, the true incidence of pediatric heart failure is unknown. One study estimates that approximately 6,000 patients younger than 19 years of age visit an ED annually in the United States for a heart failure-related complaint among an estimated 12,000 to 35,000 total children living in the United States with heart failure each year.2,3 By comparison, about 800,000 such visits occur annually in the adult population.6 Nearly half of ED visits by pediatric patients with heart failure are by those younger than 1 year of age, with no major differences in prevalence between males and females.3 Additionally, the frequency of VAD implantation in children with severe heart failure is increasing, and select centers have started to transition VAD-supported children to outpatient care.7-9
History and Physical Exam
The major challenge ED physicians face in diagnosing new-onset heart failure in children is differentiating nonspecific complaints that mirror common, alternative diagnoses. The difficulty with early identification of heart failure was highlighted in a recent study, which reported that 49% of patients were diagnosed incorrectly at their initial presentation to the ED.10 Common incorrect diagnoses included pneumonia, viral syndrome, gastroenteritis, asthma, and constipation. Moreover, half of providers failed to document cardiac disease in their differential diagnosis. Additionally, a detailed history and physical exam are crucial to guide further diagnostic workup for children with potential new-onset cardiac dysfunction, because errors in history or physical exam account for half of missed diagnoses.
Signs and symptoms of patients with new-onset heart failure largely depend on age. Parents of infants might describe fatigue, vomiting, color change, weight loss, or sweating with feeds.10 Historical indicators in verbal patients include chest pain, shortness of breath, abdominal pain, syncope, or exertional fatigue. Physical exam findings include the presence of a cardiac murmur or gallop; signs of left ventricular (LV) failure or aortic outflow obstruction (unexplained tachycardia, tachypnea, signs of respiratory distress, and/or rales on auscultation); signs of hypoperfusion (pallor, delayed capillary refill time, diminished pulses, and/or cool extremities); and signs of right ventricular (RV) failure (elevated jugular venous pressure and/or hepatomegaly). No single feature obtained in the history or physical exam is diagnostic, but a combination of findings is worrisome and should be explored further.
For children with known heart failure, physicians should explore for potential complications with history and physical exam. As with patients in new-onset heart failure, patients with exacerbations of existing heart failure can have nonspecific symptoms, including fatigue, emesis, and difficulty feeding.
Older patients might report worsening dyspnea, orthopnea, or exertional complaints. Of note, some features of volume overload in adults, such as weight gain, are not reliable in children because of ongoing growth and frequency of associated vomiting.11 Infants and young children also are less likely to have lower extremity edema.12 Furthermore, because nearly half of known heart failure patients have congenital heart disease (CHD), questions regarding surgical history should be asked.
Physicians also should ask about current medication regimens, including use of anticoagulants, antiarrhythmics, afterload reducers, and diuretics, as well as any recent difficulties with medication adherence. If applicable, physicians should inquire about complications of medical therapy, including neurologic complications or gastrointestinal bleeding. Finally, patients with VADs are a unique population that will be discussed later in this article.
Differential Diagnosis
New-onset heart failure is a rare diagnosis among pediatric patients evaluated in the ED. Thus, on most occasions, nonspecific complaints will be due to an alternative cause. For example, causes of vomiting include gastroenteritis, appendicitis, and testicular or ovarian torsion. Tachypnea might be due to a respiratory illness, pneumonia, or foreign body aspiration. Altered mental status and poor perfusion, while present in fulminant myocarditis, also are signs of shock. Therefore, cardiac disease requires a high index of suspicion among pediatric patients with nonspecific complaints to decrease the chance of a missed diagnosis.
Case-Based Introductions to Covered Topics
Case #1: Child with Acute Heart Failure
A male aged 1 year with no past medical history presents to the ED with lethargy. His parents report that he had nasal congestion, cough, and fever two weeks prior. He developed increasing lethargy during the past two days with associated difficulty breathing. His vital signs include a heart rate of 190 beats/min, blood pressure of 60/40 mmHg, a respiratory rate of 50 breaths/min, and pulse oximetry of 88% on room air. On exam, he appears lethargic, pale, and tachypneic, with hepatomegaly, cool extremities, diminished pulses, and delayed capillary refill time.
Definitions
Heart failure is a manifestation of numerous disease processes within the pediatric population. Broadly, causes of pediatric heart failure include acquired heart disease (including tachyarrhythmias), congenital structural heart disease, and intrinsic disease of the myocardium. All result in impaired ventricular filling during diastole, ejection of blood during systole, or both. Patients in severe heart failure may present in cardiogenic shock, defined as a state of inadequate tissue oxygenation due to decreased cardiac output.
Clinical Features
Acquired Heart Disease. Myocar-ditis, rheumatic heart disease, and cardiotoxicity related to drug use all are examples of acquired cardiac disease that may result in heart failure. Acquired forms of heart failure also include tachycardia-induced cardiomyopathy and high-output cardiac failure.
Myocarditis is an inflammatory disease of the myocardium that has numerous causes. The most common etiology is due to a number of implicated viruses, including coxsackievirus B, adenovirus, influenza, Epstein-Barr virus, cytomegalovirus, hepatitis C, and human herpesvirus 6.13 Trypanosoma sp. (Chagas disease), Rickettsia sp. (Rocky Mountain spotted fever), and Borrelia sp. (Lyme disease) also have been reported as causative infectious agents.13,14 Additionally, immune-mediated conditions, such as Kawasaki disease, might cause myocarditis. Myocarditis occurs in a bimodal age distribution and affects children younger than 2 years and teens aged between the ages of 13 and 18 years more frequently than children in other age groups.
In a recent multicenter cohort study, no single historical feature or physical exam finding was present in a majority of children.15 Findings included chest pain, gastrointestinal symptoms, a viral prodrome, respiratory distress, hepatomegaly, a cardiac gallop, and signs of poor perfusion.
To highlight the diagnostic challenge of acute heart failure, 47% of hospitalized patients diagnosed with myocarditis had no significant physical exam findings on initial presentation.15
Rheumatic heart disease is rare in the United States, yet it is the most important form of acquired heart disease in children living in developing countries. Rheumatic heart disease is an inflammatory process in structures of the heart that typically begins two to three weeks following infections with Streptococcus sp.16 The time to symptom onset following the development of rheumatic heart disease is highly variable, and symptoms might not occur for several years and into adulthood.
Anthracyclines (e.g., doxorubicin or daunorubicin) are well-known cardiotoxic chemotherapeutic agents used in several oncologic treatment regimens. Patients with histories of malignancy, particular those with concomitant radiation therapy, are at risk for acute heart failure.17 Anthracycline cardiotoxicity also is dose-dependent, with an increased risk of heart failure at higher cumulative exposure.
Classifications of early cardiotoxicity (immediately following exposure and reversible), subacute cardiotoxicity (within a year of exposure), and late cardiotoxicity (occurring years after exposure) exist, but prospective data on the prevalence and disease course are limited. As such, children with drug-related cardiotoxicity may present with acute heart failure virtually any time after treatment.17
Tachycardia-induced cardiomyopathy is an acquired form of heart failure that, while rare, is potentially curable. Examples of disorders that cause tachycardia-induced cardiomyopathy include sustained tachycardia syndromes, such as atrial ectopic tachycardia (AET), which is the most common; focal atrial tachycardia; and permanent junctional reciprocating tachycardia. Paroxysmal tachycardia syndromes, such as variants of supraventricular tachycardia, also may lead to heart failure. The pathophysiology of tachycardia-induced cardiomyopathy is multifactorial, with mechanisms such as myocardial ischemia, structural remodeling, and loss of contractility due to myocyte structural malalignment and rapid calcium cycling all contributing.18
Tachycardia syndromes can occur at any age. The timing of heart failure symptoms following the onset of tachycardia also is variable. As such, clinical features can range from nonspecific signs of heart failure in young infants to palpitations and/or exertional dyspnea in older children.
High-output cardiac disease is a rare cause of acquired heart failure in children. It is characterized by a high cardiac output (CO) and low systemic vascular resistance (SVR) state. Causes include metabolic diseases, such as hyperthyroidism; severe thiamine deficiency, such as in beriberi; and arteriovenous fistulas, such as in McCune-Albright syndrome or hereditary hemorrhagic telangiectasia. Children may present with sequelae of the underlying disease process in addition to heart failure.
Structural Heart Disease. Stru-ctural heart disease is the most common etiology for pediatric heart failure and includes the spectrum of CHD syndromes (e.g., tetralogy of Fallot, single ventricle, systemic right ventricle, valvular defects, septal wall defects, and coronary artery anomalies). Heart failure in structural heart disease is the end result of altered pressures within the chambers of the heart due to anatomic abnormalities, abnormal systemic-pulmonary connections (shunting), or as a result of ischemia, as with coronary artery anomalies. Patients with structural heart disease often are diagnosed in-utero, and most are diagnosed within the first year of life.2 However, specific disease processes tend to result in symptoms within general timeframes. For example, patients with ductal-dependent obstructive cardiac abnormalities, such as aortic coarctation, critical aortic stenosis, and hypoplastic left heart syndrome, typically present between 2 and 14 days of age as the ductus begins to close.
Clinical manifestations in patients with obstructive disease include cyanosis, tachypnea, decreased pulses, or an abnormal blood pressure gradient between the upper and lower extremities. As another example, patients with coronary artery anomalies may present at any age, but those with an anomalous left coronary artery from the pulmonary artery (ALCAPA) usually present between 2 and 6 months of age.19,20 In ALCAPA, ischemia of the myocardium in the left coronary artery territory results from an aberrant supply of low-pressure, deoxygenated blood from the pulmonary artery. Symptoms occur in patients with ALCAPA as pulmonary vascular resistance drops during normal infant development and ischemia worsens.
However, as with all patients with heart failure due to structural heart disease, the timing and severity of presentation depend on the type of abnormality and the presence of collateral circulation. Patients with mild abnormalities or sufficient collateral circulation might remain asymptomatic for years and can present later in life with chest pain, anginal equivalents, exertional syncope, or sudden cardiovascular collapse.
Intrinsic Heart Disease. The list of inherited disorders of the myocardium is expansive. Examples include muscular dystrophies, glycogen storage diseases, fatty acid oxidation disorders, disorders of carnitine uptake, and genetic mutations of myocardial proteins, such as troponin.21 All may result in cardiomyopathy, further divided into hypertrophic, dilated, or restrictive subtypes and subsequent heart failure. Children with intrinsic heart disease may present at any age, depending on the specific etiology. Many of these disorders have strong familial associations or genetic inheritance patterns, and symptomatic children with a positive family history should prompt consideration of further evaluation. In addition to signs of heart failure, children with intrinsic diseases of the myocardium are prone to dysrhythmias, syncope, and sudden cardiac arrest.21,22
Cardiogenic Shock. Children in acute heart failure are more likely than adults to present in fulminant cardiogenic shock. Because of a predominance of stiff myocardial collagen fibers, resulting in a less-compliant heart, young children cannot augment contractility and stroke volume as well as adults in times of increased physiological stress.
Instead, young children rely on increases in heart rate and SVR to compensate. However, increased afterload, due to a rise in SVR, drops CO more and can create a low-CO shock state. Furthermore, myocardial cells in young children lack well-developed sarcoplasmic reticulum, which is responsible for intracellular calcium storage and release for cardiac contraction. Due to a lack of intracellular calcium storage, cardiac contraction in young children is more reliant on extracellular calcium concentrations to maintain CO than in adults.
As compensatory mechanisms fail in cardiac disease, the combination of low CO and high SVR results in a loss of tissue perfusion and end-organ dysfunction. Clinical signs of cardiogenic shock in pediatric patients include altered mental status, abnormal skin perfusion (cold shock, delayed capillary refill time, cool extremities, and/or mottling), diminished quality of pulses, low blood pressure, and low urine output
(< 1 mL/kg/hour). Tachycardia also is an important finding, but it is not reliable in children because of the multitude of factors affecting heart rate, such as agitation and fever.
Diagnostic Evaluation. Unfortu-nately, no well-established criteria or decision tools exist to guide diagnostic evaluation for possible heart failure in children with nonspecific symptoms presenting to the ED. Providers must weigh the possibility of the rare patient with cardiac disease against the drawbacks of overtesting. However, patients with a combination of symptoms and physical exam findings concerning for heart failure should undergo further assessment. Patients with known heart disease also should undergo testing if the clinical presentation warrants. Additionally, evaluation and empiric treatment in critically ill patients should initially, or at least simultaneously, address the more common etiologies of shock, such as sepsis, rather than heart disease alone.
Electrocardiogram. The electrocardiogram (ECG) is noninvasive, rapid, and useful as a screening test in pediatric patients with possible heart failure. While ECG findings are variable and often nonspecific, they usually are abnormal in the setting of significant disease. In myocarditis, sinus tachycardia is the most common abnormality. Others include conduction delay, ventricular ectopy, ventricular dysrhythmias, and T-wave abnormalities.5,23 (See Figure 1.) If myocarditis involves the pericardium (myopericarditis), the ECG may demonstrate widespread ST elevations and PR depressions, with reciprocal ST depression and PR elevation in lead augmented vector right (aVR).
Figure 1. Electrocardiogram Showing Myocarditis |
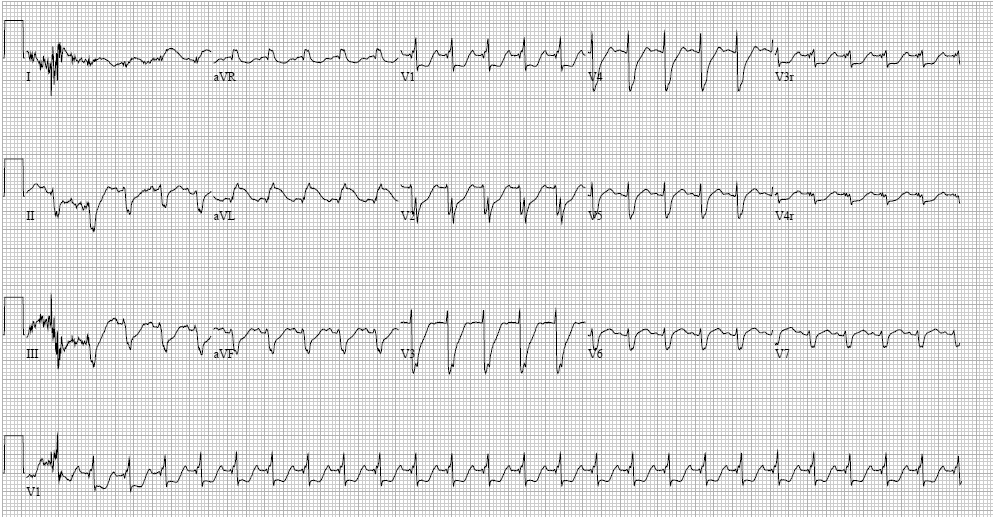 Image courtesy of Matthew Murray, MD. |
In another example, dilated cardiomyopathy results in enlarged atria and dysfunction of cardiac conduction, which translates to enlarged P waves, prolonged QRS intervals, or bundle-branch block. Additionally, dagger-like inferior (II, III, augmented vector foot [aVF]) and lateral (I, augmented vector left [aVL], V4-V6) Q waves, left axis deviation, and widespread repolarization abnormalities are characteristic of hypertrophic cardiomyopathy.24 Myocardial ischemia due to ALCAPA generates ST changes in the anterolateral distribution (I, aVL, V5-V6), although 20% to 45% of patients do not have pathologic Q waves.25 Furthermore, the ECG in patients with AET demonstrates abnormal P-wave morphology with regular QRS intervals and depends on the origination of the electrical focus. The list of specific ECG abnormalities related to heart failure is extensive and beyond the scope of this article.
Chest X-Ray. Chest X-ray also is useful as a screening test for heart failure in pediatric patients, as well as to evaluate for signs of worsening failure in those with known disease. Cardiomegaly, defined as cardiac size greater than half of the width of the chest on X-ray, had a negative predictive value of 91.1% and specificity of 92.3% in predicting ventricular dilation on echocardiogram in a cohort of children referred to a pediatric cardiology clinic.26 (See Figure 2.) Other findings on chest X-ray include pulmonary vascular congestion and pulmonary edema.27
Figure 2. Chest X-Ray Showing Cardiomegaly |
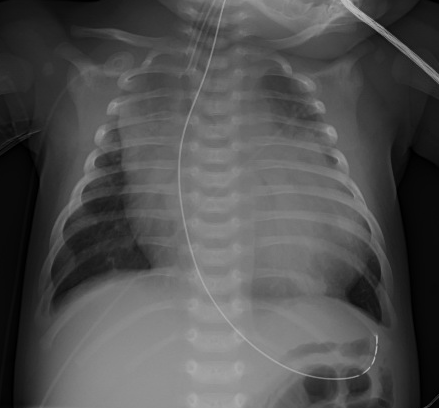 |
|
Image courtesy of Matthew Murray, MD. |
Point-of-Care Ultrasound. Emergency physicians routinely perform point-of-care ultrasound (POCUS) in the ED setting. POCUS features of heart failure include ventriculomegaly and decreased systolic function. In case reports, POCUS aided in more rapid identification of cardiac disease in pediatric patients presenting to the ED.28,29 However, a major disadvantage of POCUS is the high degree of operator expertise on diagnostic yield. Furthermore, while some evidence demonstrates a high degree of agreement between emergency clinicians and echocardiographers in determining ventricular function, evidence on POCUS is limited in the pediatric population overall and should not replace formal echocardiography.30,31
Complete Blood Count. While the complete blood count (CBC) is nonspecific, it is useful in context. For example, children with cardiac dysfunction and significant anemia identified on CBC might require blood transfusion. Prominent leukocytosis, along with elevations in other markers, such as procalcitonin in the appropriate clinical context, also might point to a different diagnosis.
Complete Metabolic Panel. A complete metabolic panel (CMP), including liver enzymes, can provide indirect evidence of cardiac disease and reveal important comorbidities. Renal dysfunction is common among pediatric patients in heart failure and is an independent risk factor for mortality.32 Elevations in aspartate transaminase (AST) and alanine transaminase (ALT) occur with hepatic congestion from right heart failure. Hyponatremia also is common in pediatric heart failure, and other electrolyte disturbances, such as hypokalemia or hypomagnesemia, might contribute to dysrhythmias due to adverse effects on cardiac conduction.
Troponin. Cardiac troponins are intracellular proteins that mediate interaction between actin and myosin within the myocardium. An elevated serum cardiac troponin reflects the release of intracellular contents from myocardial damage. Thus, etiologies of heart failure, such as myocarditis, can be detected via a rise in serum troponin. Among patients diagnosed with myocarditis, at least 81% have an elevated troponin.33,34
However, elevated cardiac troponin lacks specificity in pediatric patients, with a primary cardiac etiology responsible for only 46.7% of elevated troponin assays in one retrospective study.35
Brain Natriuretic Peptide and N-Terminal Pro-Brain Natriuretic Peptide. Brain natriuretic peptide (BNP) and its parent pre-hormone N-terminal pro-BNP (NT-proBNP) rise during cardiac stress. For example, BNP has a reported sensitivity between 71% and 93% and specificity between 63% and 95% to detect new or worsening structural and functional cardiac disease in children.36
There are no guidelines that favor the use of one over the other, and in practice, these markers are used interchangeably. However, there are important differences between NT-proBNP and BNP. Thresholds that suggest heart failure differ between the two tests and, thus, limit clinicians’ abilities to compare one to the other.
NT-proBNP and BNP also are markedly elevated at birth and normalize around age 1 week.36 In addition, NT-proBNP is removed almost entirely via the kidneys, and false elevations can occur with severe renal dysfunction.37 However, BNP is cleared via receptor binding and endopeptidases at multiple sites and is less affected by poor renal function. Assay choice often is limited to availability at the institution and should be interpreted with the information discussed earlier in mind.
ED Management and Disposition
For stable patients with features that warrant further diagnostic evaluation, a stepwise approach starting with noninvasive measures, including ECG and chest X-ray, is reasonable. Providers should consider laboratory testing in patients with high clinical concern of heart failure, an abnormal ECG, or radiographic signs of heart failure, such as cardiomegaly or pulmonary edema. Concerning features, such as enlarged chamber size or reduced ejection fraction on POCUS, also may aid in diagnosis early in management.
In patients with suspected heart failure, consultation with a pediatric cardiologist and formal echocardiography is warranted. Because pediatric patients may have low- or high-volume status in the setting of acute heart failure, judicious volume repletion or administration of a loop diuretic, such as furosemide, in consultation with a pediatric cardiologist may be necessary. Most patients with new-onset heart failure require admission. (See Figure 3.) Patients with known heart failure presenting with symptoms suggestive of worsening cardiac function or volume overload also should undergo laboratory and imaging testing. Pediatric patients presenting with mild volume overload may be discharged with medication adjustments in coordination with pediatric cardiology, depending on family comfort and ability for close follow-up. Signs of worsening cardiac function or significant volume overload generally warrant admission to a pediatric cardiology service for further evaluation and treatment.
Figure 3. Emergency Department Evaluation, Treatment, and Disposition of Pediatric Patients with Suspected Acute Heart Failure |
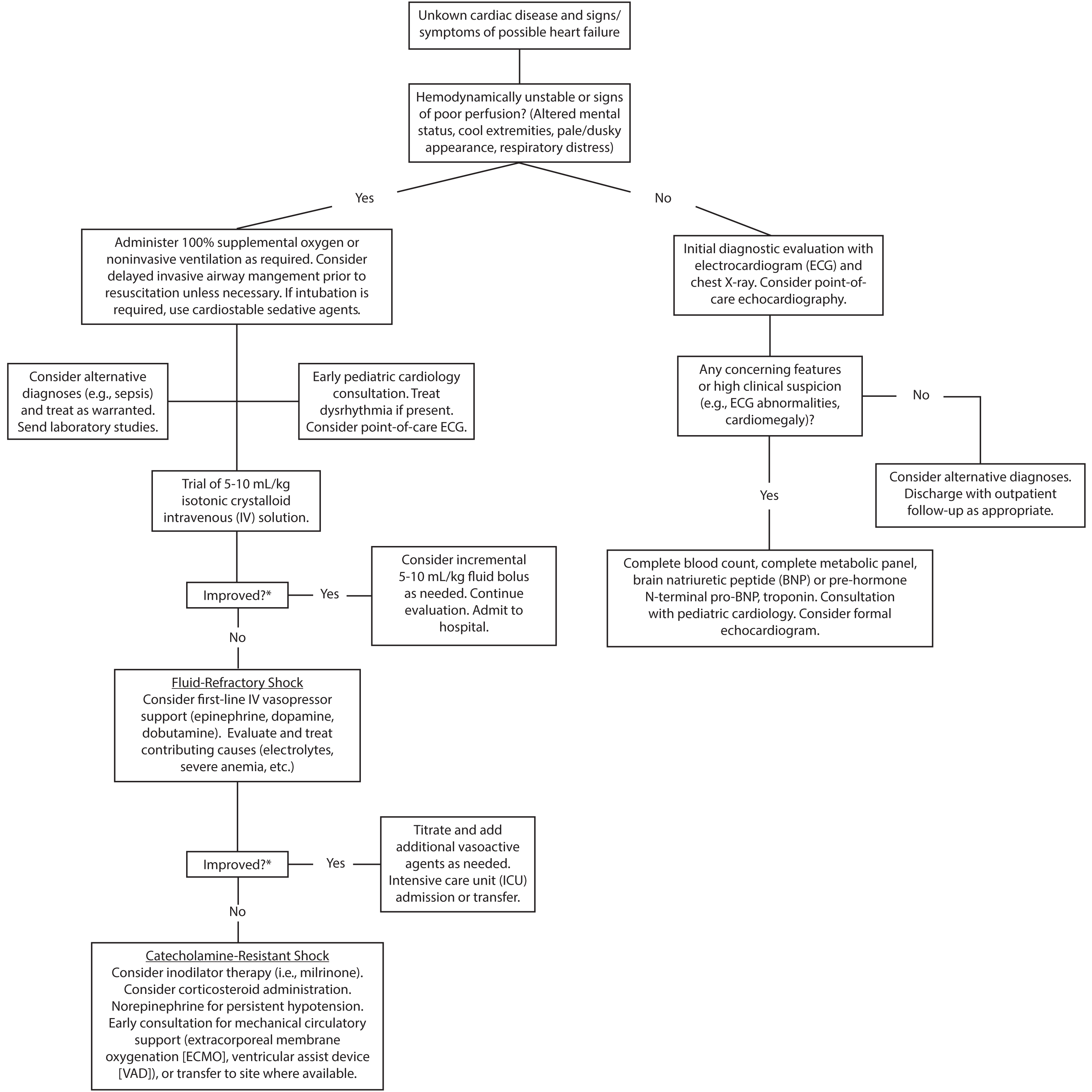 |
|
*Clinical targets for improvement include blood pressure, capillary refill time, skin temperature, quality of pulses, and mental status. Heart rate is a less reliable indicator in pediatric patients due to high variability and influence by multiple factors. |
For unstable patients in shock, resuscitation following Pediatric Advanced Life Support (PALS) guidelines takes priority. Because cardiogenic shock is rare compared with other etiologies of shock, such as sepsis, a broad differential is important. Rapid intravenous or intraosseous access should be obtained. Measures such as early broad-spectrum antibiotic administration should be strongly considered in an undifferentiated patient given the benefit in sepsis mortality.38,39
Furthermore, clinical, rather than laboratory, markers should guide ED providers during resuscitation of pediatric patients in shock. (See Figure 3.) Unstable patients with undifferentiated shock require frequent reassessment until a cause is identified and a sustained trajectory of clinical improvement occurs.
Airway Management. All patients in shock should receive ventilatory support, if necessary, as well as supplemental oxygen.40 While mechanical ventilation has benefits, such as reduction in metabolic demand and afterload reduction, patients in shock are at risk of cardiovascular collapse during intubation due to adverse hemodynamic effects of induction agents and initial preload reduction.40-42 Thus, providers should consider hemodynamic optimization prior to invasive airway management if intubation is not required immediately. If intubation is necessary, induction agents with minimal cardiac depressant effects, such as ketamine, are preferable.41 Providers also should consider having weight-based doses of epinephrine rapidly available for use in the event of cardiovascular collapse following intubation.
Treat Dysrhythmias if Present. Children with acute heart failure are at risk for dysrhythmias. Unstable patients with tachydysrhythmias should be treated with immediate synchronized cardioversion at 0.5 to 1 J/kg, or as per PALS guidelines.43 Providers should consider consultation with a cardiology specialist for refractory dysrhythmias.
Volume Administration. If cardiogenic shock is suspected, an initial isotonic crystalloid bolus of
5 mL/kg to 10 mL/kg, rather than
20 mL/kg, should be considered, with serial reassessments to evaluate for signs of worsening heart failure (e.g., worsening hemodynamic parameters, hepatomegaly, and/or pulmonary rales).41
If clinical parameters (e.g., mental status, blood pressure, and/or skin perfusion) improve after volume administration, incremental
5 mL/kg to 10 mL/kg boluses can be considered. If signs of worsening heart failure occur with volume administration, additional fluids should be held, and diuretics should be considered in consultation with a pediatric cardiologist.
Identifying Metabolic Abnormalities. Identification of metabolic abnormalities that contribute to the effects of shock is vital. POC blood gas and glucose testing can rapidly identify electrolyte abnormalities and hypoglycemia when present. Given the reliance on extracellular calcium in young children as previously discussed, correction of hypocalcemia may improve cardiac contractility and CO.
Fluid-Refractory Shock. Chil-dren with worsening or unresponsive clinical parameters following volume administration should be started on vasoactive agents.41 The goal of vasoactive drug therapy is to improve tissue perfusion by increasing CO and augmenting SVR. Appropriate first-line agents include epinephrine, dopamine, and dobutamine. Epinephrine at doses less than
0.3 mcg/kg/min favors beta agonism, which results in increased heart rate (↑CO) and peripheral vasodilation (↓SVR).
Doses more than 0.3 mcg/kg/min increase alpha agonism, causing vasoconstriction (↑SVR). Some authors favor the use of epinephrine as a first-line agent because of its ability to increase CO and reduce SVR, as well as evidence of improved outcomes when compared with the use of dopamine in pediatric fluid-refractory septic shock. 44-46
Catecholamine-Resistant Shock. Providers should consider the addition of inodilator agents in normotensive pediatric patients with continued abnormal clinical parameters despite vasopressor use.
Milrinone is a long-acting phosphodiesterase inhibitor that has a synergistic effect with beta-agonists like epinephrine, increases cardiac contractility (↑CO), and causes vasodilation (↓SVR). Doses of a milrinone infusion range from
0.25 mcg/kg/min to 0.75 mcg/kg/min. Providers should be cautious to bolus milrinone, given its long half-life and potential for hypotension, or administer it at all if the patient is persistently hypotensive.41
Hypotension can be improved with the addition of norepinephrine. Providers also should consider the addition of corticosteroid administration in patients with
catecholamine-resistant shock or those with known adrenal insufficiency, although there are minimal data to support use specifically in cardiogenic shock.
Mechanical Circulatory Support. Mechanical circulatory support includes extracorporeal membrane oxygenation (ECMO) and VAD placement. The goal of mechanical circulatory support is to provide patients with a bridge to recovery or cardiac transplantation. Early consideration should be given to mechanical circulatory support, or rapid transfer to a center capable of such support, in any unstable patient with suspected acute heart failure, particularly those not responding to volume administration or vasoactive therapy.41,47 ECMO also has been used successfully in select pediatric patients in cardiac arrest.48,49 In addition, VAD device use in heart failure is associated with increased survival when compared with ECMO, but use is limited to highly specialized centers and is not widely available.50,51
Case #2: Child with a VAD
A female 14 years of age presents with fatigue after a recent hospitalization for cardiogenic shock due to myocarditis requiring escalating medical therapy followed by VAD placement. She reports fatigue during the last three days with associated yellowing of her eyes and dark-colored urine. Her vital signs include a heart rate of 100 beats/min, mean arterial pressure (MAP) of 60 mmHg, respiratory rate of 22 breaths/min, and pulse oximetry of 98% on room air. You hear harsh grating sounds on auscultation and note high power requirements upon inspection of the device.
VAD History and Definitions
Implantation of VADs, which increase blood flow in patients with severe heart failure, started in the 1960s as a form of mechanical circulatory support while awaiting transplant. VADs are powered via drivelines that exit the thorax and connect to a device controller and battery supply.
Left ventricular assist devices (LVADs) are by far the most common. They intake blood from a cannula inserted in the left ventricle and provide either continuous or pulsatile blood flow directly into the aorta. Right ventricular assist devices (RVADs) similarly assist RV function. Biventricular assist devices (BiVADs) aid both pulmonary and systemic blood flow. These devices also can automatically adjust rates for increased demand.
VAD placement is indicated for heart failure patients refractory to medical therapy as a bridge to transplant, recovery, or to palliate severe symptoms. Previously, VAD technology was limited to adult-size patients due to size constraints, but the smaller size of newer-generation VADs has allowed use in children. Between 2012 and 2017, a total of 508 devices, 81% of which were LVADs, were implanted across 30 U.S. hospitals.52
The only pediatric-specific VAD currently approved by the Food and Drug Administration is the Berlin Heart EXCOR, a pulsatile flow device with an external pump. Other devices, such as the HeartWare HVAD System, a continuous flow device, also are in use.53 Pediatric patients may live with VADs for weeks to more than a year after placement while awaiting transplant.
Clinical Features
Pediatric patients with VADs are at risk for a unique set of associated complications that require an understanding of device terminology. (See Table 1.)
Table 1. Definitions of Important Terms Involving Ventricular Assist Devices |
|
|
Term |
Definition |
|
Pump power |
Measurement of voltage applied to rotor; varies with flow |
|
Revolutions per minute (RPM) |
Component of device flow. |
|
Flow |
Measurement of continuous blood flow generated by the device. |
|
Pulsatile index (PI) |
Degree of blood flow generated through cardiac contraction. |
|
Driveline |
Cable connecting the internal pump to the external controller. |
|
Suction event |
Event in which the ventricular wall contacts the inflow cannula. |
Unique Aspects of Patient Assessment. In patients with a VAD, assessment includes both the patient and the device itself. Evaluation of the patient also differs from that of the general population. In patients with a continuous flow device, the constant hum of the device limits auscultation for heart sounds and eliminates the ability to measure blood pressure with an automated cuff. Instead, a cardiac monitor can be used to determine cardiac rate and rhythm. In addition, the initial sounds produced by a Doppler overlying the brachial artery during release of a manual blood pressure cuff indicate a MAP in patients with a continuous flow device.
Furthermore, all patients should undergo interrogation of the device controller for the presence of alarms and changes to baseline measurements of power, flow, and pulsatile index (PI). Examination also should consist of inspection of driveline exit sites for signs of infection or damage.
Laboratory and imaging studies also are useful to assess for VAD complications in stable patients. Early consultation with an advanced pediatric heart failure specialist or VAD care coordinator helps guide evaluation.
Specific Presenting Complaints. Patients with VADs experience both device- and non-device-related complications that result in a various clinical features.54-56 (See Table 2, available at https://bit.ly/2YMVWIU.).
Among patients listed in a national registry, 70% with pulsatile flow devices and 55% with continuous flow devices had at least one adverse event during the life of the device.56 Patients with adverse events might have device alarms with or without various clinical symptoms, including device failure and circulatory arrest.
Device Alarms. The device controller of a VAD alarms with both visual and auditory stimuli in the context of abnormal function. Alarms indicate impending or existing failure of external components (e.g., batteries and/or controller) or internal components (pump), or episodic dysfunction, such as during a suction event. Device readouts during alarms vary somewhat by type of VAD, but they generally are separated into red or yellow alarms, indicating an emergent life-threatening hazard or urgent (but not immediately threatening) problem, respectively. For example, a low battery in a HeartWare HVAD results in a yellow advisory alarm. A red hazard alarm might occur following damage, or pump or controller failure.
Suction events occur in low-preload or high-flow states, when reduced ventricular filling or excessive pump speeds precipitate suction of the ventricular wall into the VAD cannula. Patients with device alarms have a varying degree of symptoms, ranging from being asymptomatic to presenting with fulminant cardiovascular collapse.
Shortness of Breath. Dyspnea and increased work of breathing are worrisome clinical findings in pediatric VAD patients.
In younger patients, shortness of breath also may manifest as decreased activity or tachypnea. Causes of shortness of breath include worsening LV function, RV failure, pericardial effusion, device thrombosis, and anemia. Worsening LV function or RV failure commonly occurs in VAD patients. Failure to thrive, difficulty feeding, abdominal pain, vomiting, tachycardia, hepatomegaly, and elevated jugular venous pressure (JVP) are co-occurring findings.57 Tachycardia, elevated JVP, and lightheadedness also are findings in patients with a pericardial effusion.
Patients also are at risk of VAD thrombosis, or partial to complete occlusion of the device, even if anticoagulated within therapeutic range.58 Signs and symptoms depend on the degree of device thrombosis and, aside from shortness of breath, include lightheadedness, tachycardia, and low blood pressure. Rough, grating noises (as opposed to a mechanical humming noise) also might be present on auscultation in patients with device thrombosis. Furthermore, VAD patients also commonly develop anemia due to increased hemolysis, and severe anemia may present with dyspnea.
Chest Pain. There is little available literature on the causes of chest pain in pediatric VAD patients. In adults with VADs, however, chest pain typically is due to a noncardiac cause.55 Noncardiac causes are extensive and include pneumonia, pneumothorax, pericarditis, and gastroesophageal reflux disease. Aortic dissection, device infection, and pericarditis are possible cardiac causes of chest pain in VAD patients.
Lightheadedness or Syncope. Low CO or device flow states result in lightheadedness or syncope. A common reason is hypovolemia, which may be due to bleeding, a recent gastrointestinal illness, or a recent increase in diuretics. In hypovolemia, a low device output develops due to a lack of ventricular preload for the device to intake. Another possibility is development of a ventricular dysrhythmia, which may be precipitated by a suction event.
Fatigue. Fatigue is a nonspecific clinical finding in a variety of conditions. Clinically important diagnoses that cause fatigue are anemia and device thrombosis. As discussed earlier, patients with VADs are at risk for anemia due to hemolysis from the device. Device thrombosis increases hemolysis, and additional signs include jaundice, scleral icterus, dark-colored urine, and shortness of breath.58
Bleeding. All patients with VADs require anticoagulation, typically with warfarin, and are accordingly at risk for acute hemorrhage. Overall, about one-third of pediatric VAD patients experience major bleeding, defined as bleeding requiring hospitalization, surgery, transfusion, or resulting in death.56
Manifestations of bleeding include melena, hematochezia, and intracranial hemorrhage. Furthermore, bleeding also may occur near cannula insertion sites resulting in pericardial effusion and tamponade.
Acute Neurologic Complaints. Neurologic complications also are frequent in pediatric VAD patients.56 Stroke results from embolism of device-associated thrombosis or acute intracranial hemorrhage. Acute alterations in mental status, severe headache, focal weakness, and speech abnormalities are potential clinical manifestations.
Signs of Infection. Pediatric patients with VADs are at high risk to develop a device-associated infection at some point during the life of the device.56 Device-associated infections occur around the driveline exit site, pump pocket, and pump itself.
Related infections include endocarditis, mediastinitis, and bacteremia. Clinical findings include fever, chest pain, shortness of breath, erythema or discharge from driveline exit sites, and signs of sepsis.
Circulatory Arrest. Patients with a continuous flow device lack pulses at baseline, but when they are in circulatory arrest, they will be unresponsive, lack respiratory effort, and have signs of poor perfusion (e.g., delayed or absent capillary refill, and/or cool extremities). Among patients in circulatory arrest due to device failure, sounds of the device will not be appreciated on auscultation.
Diagnostic Evaluation
Device Interrogation. A crucial component of the diagnostic evaluation of pediatric VAD patients is device interrogation. The device controller should be checked for ongoing alarms and alarm history. Alarm prompts on the device also generally provide specific direction of what to do next.
For example, a yellow advisory alarm due to a low battery in the HeartWare HVAD also displays a message to replace the battery. Other alarms prompt the user to call for help. However, a general understanding of device function helps understand specific problems.
A low flow alarm is due to a device flow below a set threshold and results from inadequate blood volume intake (e.g., kinked inflow cannula, low volume state, RV failure, and/or effusion/tamponade), obstruction within the device (thrombosis), or a problem with blood outflow (hypertension and/or kinked outflow catheter).
High power alarms may result from device compensation to overcome inadequate flow, such as in device thrombosis.
Controller failure or device stoppage alarms result from device malfunction or disconnection of components.
Furthermore, device values outside the normal range can provide a clue to the diagnosis. A lower-than-
normal PI, for example, can be due to a low volume state. Low power levels can be due to driveline kinking or fraying. Patients in a vasodilatory state, such as septic shock, will have a high device flow rate.
ECG. The ECG is a useful tool to evaluate for dysrhythmias in pediatric patients with VADs, particularly those with recent suction events or signs of worsening heart failure.
Chest X-Ray. Pulmonary edema, pleural effusions, and increased heart size are signs of worsening heart failure on chest X-ray. Chest X-ray also might demonstrate kinking or fraying of the driveline or malposition of VAD cannulas.
POCUS. There is no evidence on the reliability or diagnostic utility of ED physician-performed POCUS for pediatric VAD patients. However, basic assessments for pericardial effusion, tamponade, or RV failure with POCUS in an undifferentiated hypotensive VAD patient may be useful.
CBC. A CBC may demonstrate anemia in VAD patients due to major bleeding or hemolysis from device thrombosis. Current guidelines also recommend obtaining a CBC to assess for leukocytosis in patients with signs of infection.59 Additionally, platelet count may be altered in VAD patients.
CMP. Providers should obtain a CMP during evaluation of VAD patients. Diminished renal function is a sign of pump thrombosis, RV failure, or worsening LV failure. Elevated liver enzymes suggest hepatic congestion and RV dysfunction. Electrolyte abnormalities contribute to the development of cardiac dysrhythmias.
Coagulation Studies. Because all patients with VAD support require anticoagulation, providers should order studies such as an international normalized ratio (INR) to assess for an appropriate therapeutic range.59
BNP and NT-proBNP. Available adult literature supports the use of NT-proBNP and BNP levels to evaluate for signs of volume overload or worsening heart failure in adult VAD patients.60,61 However, data on use of NT-proBNP or BNP to guide management of pediatric patients with VAD support are lacking.
Lactate Dehydrogenase and Plasma Free Hemoglobin. Increased lactate dehydrogenase (LDH) and plasma free hemoglobin levels occur with device thrombosis due to an associated increase in hemolysis.62 LDH three times the upper limit of normal and plasma free hemoglobin
> 40 mg/dL are specific to pump thrombosis in adult patients, although cutoffs in children are less clear.58,62
Urinalysis. Another marker suggesting device thrombosis is the presence of hemoglobinuria because of hemolysis. VAD patients with any symptom concerning for device thrombosis should undergo urinalysis testing.
Cultures. Providers should obtain three sets of blood cultures over 24 hours in all febrile patients with a VAD, given the risk of bacterial infection.59,63 If obtaining cultures for a possible driveline site infection, both bacterial and fungal cultures at the driveline exit site should be obtained.59
ED Management and Disposition
Although the frequency of device placement and the number of patients undergoing outpatient management are increasing, minimal data exist to guide pediatric-specific ED management. Approaches centered on device parameter abnormalities, the presence of hypotension, and symptoms among adult patients with a VAD have been described.59
Patients with VADs are a complex population who all require consultation with their associated care teams to help guide ED management. Patients routinely are discharged with documentation that includes device parameters and important contact information that aid evaluation. This section will outline a problem-based ED management approach for both common and truly emergent complications.
Dysrhythmias. Patients in any tachydysrhythmia, such as ventricular tachycardia with poor device flow or hemodynamic compromise, should be cardioverted.59 Stable patients may be managed medically in consultation with cardiology.
Signs of Increased Hemolysis. Because increased hemolysis might be a sign of pump thrombosis, patients with hemolysis, particularly those with abnormal pump function, require admission and optimization of anticoagulation.59 Some centers report successful management of pump thrombosis-associated hemolysis with initiation of intravenous (IV) bivalirudin, a direct thrombin inhibitor, followed by low-dose thrombolytics if unsuccessful.64
Signs of Infection. Little evidence exists to guide specific management for pediatric patients presenting to the ED with signs of infection. Most centers initiate broad-spectrum IV antibiotics after cultures are obtained, followed by hospital admission to determine if a device-associated infection is present.63 Vasopressor agents and fluid administration to improve the hemodynamic status of the patient may be necessary if signs of septic shock are present.
Acute Neurologic Injury. For patients with a new neurologic deficit, immediate consultation with neurology, computed tomography (CT) angiography of the head and neck, and determination of INR are recommended.59
Patients with findings of hemorrhagic stroke on CT imaging should undergo immediate discontinuation and reversal of anticoagulation. All patients with a new neurologic deficit also should undergo evaluation for device thrombosis, given its association with embolic stroke. Furthermore, some patients with thromboembolic events may be considered for endovascular or thrombolytic treatment.59 Pediatric VAD patients with acute neurologic injury require admission to the cardiovascular ICU for close monitoring.
Major Bleeding. Patients who are hemodynamically unstable and have significant bleeding should be resuscitated with blood products and should undergo reversal of anticoagulation.59 Although limited data exist regarding the safety of anticoagulation reversal in pediatric VAD patients, short-term reversal in adult VAD patients appears to be safe.65,66
If bleeding from the gastrointestinal tract is present, providers should consult with pediatric gastroenterology for urgent endoscopy to evaluate the source of bleeding.
Hypotension. How to best manage hypotensive VAD patients depends on the etiology of hypotension. Hypotension in patients with a continuous flow device may occur in two device states: abnormally low or high flow.59 Vasopressor use may correct hypotension in patients with high-flow states, which occur in septic shock or other causes of vasodilation. Pediatric VAD patients hypotensive from septic shock also should be treated with early broad-spectrum IV antibiotics. Hypovolemia, device thrombosis, RV dysfunction, pulmonary embolism, cardiac tamponade, and pneumothorax all are causes of low flow and hypotension. Rapid assessment, including with echocardiography and treatment for immediately correctable causes, such as bleeding, should occur.67 Definitive therapy in unstable patients with device thrombosis or malfunction is surgery for pump exchange.
Circulatory Arrest. Little evidence exists to guide proper management of VAD-supported children. Initial management of arrest should include immediate inspection for device failure and standard PALS guidelines for cardiac arrest. 67 (See Figure 4.) The Berlin Heart EXCOR also has a backup hand pump that should be used in device failure, rather than chest compressions, unless unable to generate a pulse. Devices should be inspected immediately for loss of power supply or disconnected or kinked cables. Any obvious problems should be corrected. A pediatric cardiothoracic surgery specialist should be consulted if a device failure is present.
Figure 4. Evaluation and Management of an Unresponsive or Altered Ventricular Assist Device Patient |
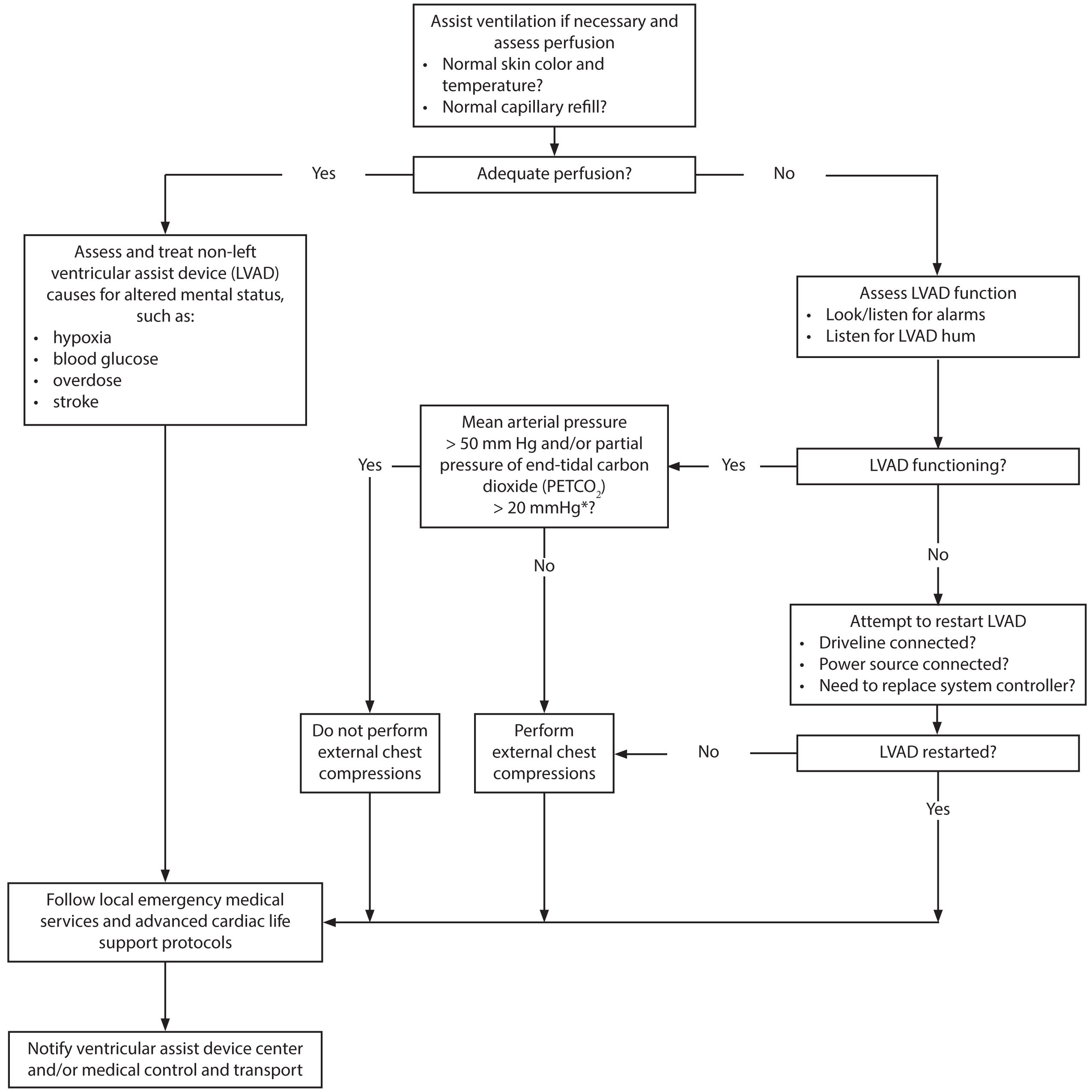 |
|
*The PETCO2 cutoff of > 20 mm Hg should be used when an endotracheal tube or tracheostomy is used to ventilate the patient. Use of a supraglottic (e.g., King) airway results in a falsely elevated PETCO2 value. |
Conclusion
Pediatric patients with heart failure are a challenging population for ED physicians. The rare incidence of heart failure combined with often nonspecific presenting complaints from patient families make diagnosis difficult.
A stepwise diagnostic evaluation is reasonable in stable patients with a combination of clinical features suggestive of heart failure to avoid unnecessary invasive testing. Providers should consider, test, and treat for alternative etiologies, such as sepsis, in hemodynamically unstable patients.
An escalating treatment approach for unstable patients in suspected heart failure includes judicious use of fluids, vasopressor use, and administration of inodilators to achieve target clinical, rather than laboratory, goals. Emergent transfer to a facility capable of mechanical circulatory support is indicated in patients with persistent hemodynamic compromise despite maximal medical therapy. Furthermore, the increased use of VAD support among children with heart failure requires an understanding of associated complications, diagnostic evaluation, and management strategies.
REFERENCES
- McDermott KW, Stocks C, Freeman WJ. Agency for Healthcare Research and Quality. Overview of pediatric emergency department visits, 2015. HCUP Statistical Briefs #242. Rockville;2018.
- Hsu DT, Pearson GD. Heart failure in children: Part I: History, etiology, and pathophysiology. Circ Heart Fail 2009;2:
63-70. - Mejia EJ, O’Connor MJ, Lin KY, et al. Characteristics and outcomes of pediatric heart failure-related emergency department visits in the United States: A population-based study. J Pediatr 2018;193:114-118.e3
- Arola A, Pikkarainen E, Sipilä JO, et al. Occurrence and features of childhood myocarditis: A nationwide study in Finland.
J Am Heart Assoc 2017;6:e005306. - Cooper LT. Myocarditis. N Eng J Med 2009;360:1526-1538.
- Blecker S, Ladopo JA, Doran KM, et al. Emergency department visits for heart failure and subsequent hospitalization or observation unit admission. Am Heart J 2014;168:90190-8.
- Villa CR, Khan MS, Zafar F, et al. United States trends in pediatric ventricular assist implantation as bridge to transplantation. ASAIO J 2017;63:470-475.
- Mehegan M, Oldenburg G, Lantz J. Pediatric VAD discharge and outpatient care. ASAIO J 2018;64:e156-e160.
- Shugh SB, Riggs KW, Morales DLS. Mechanical circulatory support in children: Past, present, and future. Transl Pediatr 2019;8:269-277.
- Puri K, Singh H, Denfield SW, et al. Missed diagnosis of new-onset systolic heart failure at first presentation in children with no known heart disease. J Pediatr 2019;208:258-264.e3.
- Del Castillo S, Shaddy RE, Kantor PF. Update on pediatric heart failure. Curr Opin Pediatr 2019;31:598-603.
- Jayaprasad N. Heart failure in children. Heart Views 2016;17:92-99.
- Kindermann I, Barth C, Mahfoud F, et al. Update on myocarditis. J Am Coll Cardiol 2012;59:779-792.
- Fung G, Luo H, Qiu Y, et al. Myocarditis. Circ Res 2016;118:496-514.
- Butts RJ, Boyle GJ, Deshpande SR, et al. Characteristics of clinically diagnosed pediatric myocarditis in a contemporary multi-center cohort. Pediatr Cardiol 2017;38:1175-1182.
- Gewitz MH, Baltimore RS, Tani LY, et al. Revision of the Jones Criteria for the diagnosis of acute rheumatic fever in the era of Doppler echocardiography: A scientific statement from the American Heart Association. Circulation 2015;131:
1806-1818. - Loar RW, Noel CV, Tunuguntla H, et al. State of the art review: Chemotherapy-induced cardiotoxicity in children. Congenit Heart Dis 2018;13:5-15.
- Huizar JF, Ellenbogen KA, Tan AY, et al. Arrhythmia-induced cardiomyopathy: JACC state-of-the-art review. J Am Coll Cardiol 2019;73:2328-2344.
- Harky A, Noshirwani A, Karadakhy O, et al. Comprehensive literature review of anomalies of the coronary arteries.
J Card Surg 2019;34:1-16. - Levitas A, Krymko H, Ioffe V, et al. Anomalous left coronary artery from the pulmonary artery in infants and toddlers misdiagnosed as myocarditis. Pediatr Emerg Care 2016;32:232-234.
- Maron BJ, Towbin JA, Thiene G, et al. Contemporary definitions and
classifications of the cardiomyopathies. Circulation 2006;113:1807-1816. - Decker JA, Rossano JW, O’Brian Smith E, et al. Risk factors and mode of death in isolated hypertrophic cardiomyopathy in children. J Am Coll Cardiol 2009;54:250-254.
- Piccininni JA, Richmond ME, Cheung EW, et al. Influenza myocarditis treated with antithymocyte globulin. Pediatrics 2018;142:e20180884.
- Veselka J, Anavekar NS, Charron P. Hypertrophic obstructive cardiomyopathy. Lancet 2017;389:1253-1267.
- Hoffman JIE. Electrocardiogram of anomalous left coronary artery from the pulmonary artery in infants. Pediatr Cardiol 2013;34:489-491.
- Satou GM, Lacro RV, Chung T, et al. Heart size on chest X-ray as a predictor of cardiac enlargement by echocardiography in children. Pediatr Cardiol 2001;22:
218-222. - Tumkosit M, Yingyong N, Mahayosnond A, et al. Accuracy of chest radiography for evaluating significantly abnormal pulmonary vascularity in children with congenital heart disease. Int J Cardiovasc Imaging 2012;28(suppl 1):s69-s75.
- Shaahinfar A, Ghazi-Askar ZM, Siroker H, et al. Anomalous left coronary artery from the pulmonary artery presenting as dilated cardiomyopathy with regional wall motion abnormality on point-of-care ultrasound. Pediatr Emerg Care 2019;35:
516-518. - Rosenfield D, Fisher JW, Kwan CW. Point-of-care ultrasound to identify congenital heart disease in the pediatric emergency department. Pediatr Emerg Care 2018;34:223-225.
- Longjohn M, Wan J, Joshi V, et al. Point-of-care echocardiography by pediatric emergency physicians. Pediatr Emerg Care 2011;27:693-696.
- Gaspar HA, Morhy SS, Lianza AC, et al. Focused cardiac ultrasound: A training course for pediatric intensivists and emergency physicians. BMC Med Educ 2014;14:25.
- Riley A, Gebhard DJ, Akcan-Arikan A. Acute kidney injury in pediatric heart failure. Curr Cardiol Rev 2016;12:121-31.
- Butto A, Rossano JW, Nandi D, et al. Elevated troponin in the first 72 h of hospitalization for pediatric viral myocarditis is associated with ECMO: An analysis of the PHIS+ database. Pediatr Cardiol 2018;39:1139-1143.
- Harris TH, Gossett JG. Diagnosis and diagnostic modalities in pediatric patients with elevated troponin. Pediatr Cardiol 2016;37:1469-1474.
- Yoldas T, Orun UA. What is the significance of elevated troponin I in children and adolescents? A diagnostic approach. Ped Cardiol 2019;40:1638-1644.
- Neves AL, Henriques-Coelho T, Leite-Moreira A, et al. The utility of brain natriuretic peptide in pediatric cardiology: A review. Pediatr Crit Care Med 2016;17:e529-e538.
- Takase H, Dohi Y. Kidney function crucially affects B-type natriuretic peptide (BNP), N-terminal proBNP and their relationship. Eur J Clin Invest 2014;44:
303-308. - Weiss SL, Fitzgerald JC, Balamuth F, et al. Delayed antimicrobial therapy increases mortality and organ dysfunction duration in pediatric sepsis. Crit Care Med 2014;42:2409-2417.
- Lane RD, Funai T, Reeder R, et al. High reliability pediatric septic shock quality improvement initiative and decreasing mortality. Pediatrics 2016;138:e20154153.
- Bronicki RA, Taylor M, Baden H. Critical heart failure and shock. Pediatr Crit Care Med 2016;17(8 suppl 1):s124-s130.
- Davis AL, Carcillo JA, Aneja RK, et al. American College of Critical Care Medicine clinical practice parameters for hemodynamic support of pediatric and neonatal septic shock. Crit Care Med 2017;45:1061-1093.
- Shiima Y, Berg RA, Bogner HR, et al. Cardiac arrests associated with tracheal intubations in PICUs: A multicenter cohort study. Crit Care Med 2016;44:
1675-1682. - Duff JP, Topjian A, Berg MD, et al. 2018 American Heart Association focused update on pediatric advanced life support: An update to the American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation 2018;138:e731–e739.
- Del Castillo S, Shaddy RE, Kantor PF. Update on pediatric heart failure. Curr Opin Pediatr 2019;31:598-603.
- Ramaswamy KN, Singhi S, Jayashree M, et al. Double-blind randomized clinical trial comparing dopamine and epinephrine in pediatric fluid-refractory hypotensive septic shock. Pediatr Crit Care Med 2016;17:e502-e512.
- Ventura AMC, Shieh HH, Bousso A, et al. Double-blind prospective randomized controlled trial of dopamine versus epinephrine as first-line vasoactive drugs in pediatric septic shock. Crit Care Med 2015;43:
2292-2302. - Rajagopal SK, Almond CS, Laussen PC, et al. Extracorporeal membrane oxygenation for the support of infants, children, and young adults with acute myocarditis: A review of the Extracorporeal Life Support Organization registry. Crit Care Med 2010;38:382-387.
- Absi M, Kumar ST, Sandhu H. The use of extracorporeal membrane oxygenation-cardiopulmonary resuscitation in prolonged cardiac arrest in pediatric patients: Is it time to expand it? Pediatr Emerg Care 2017;33:e67-e70.
- Barrett CS, Bratton SL, Salvin JW, et al. Neurological injury after extracorporeal membrane oxygenation use to aid pediatric cardiopulmonary resuscitation. Pediatr Crit Care Med 2009;10:445-451.
- Almond CS, Morales DL, Blackstone EH, et al. Berlin Heart EXCOR pediatric ventricular assist device for bridge to heart transplantation in US children. Circulation 2013;127:1702-1711.
- Fraser CD, Jaquiss RDB, Rosenthal DN, et al. Prospective trial of a pediatric ventricular assist device. N Engl J Med 2012;367:
532-541. - Morales DLS, Rossano JW, VanderPluym C, et al. Third annual Pediatric Interagency Registry for Mechanical Circulatory Support (PediMACS) report: Preimplant characteristics and outcomes. Ann Thorac Surg 2019;107:933-1004.
- Pratt AK, Shah NS, Boyce SW. Left ventricular device management in the ICU. Crit Care Med 2014;42:158-168.
- VanderPluym CJ, Adachi I, Niebler R, et al. Outcomes of children supported with an intracorporeal continuous-flow left ventricular assist system. J Heart Lung Transplant 2019;38:385-393.
- Sen A, Larson JS, Kashani KB, et al. Mechanical circulatory assist devices: A primer for critical care and emergency physicians. Crit Care 2016;20:153.
- Rosenthal DN, Almond CS, Jaquiss RD, et al. Adverse events in children implanted with ventricular assist devices in the U.S.: Data from the Pediatric Interagency Registry for Mechanical Circulatory Support (PediMACS). J Heart Lung Transplant 2016;35:569-577.
- Konstam MA, Kiernan MS, Bernstein D, et al. Evaluation and management of right-sided heart failure: A scientific statement from the American Heart Association. Circulation 2018;137:e578-e622.
- Starling RC, Moazami N, Silvestry SC, et al. Unexpected abrupt increase in left ventricular assist device thrombosis. N Engl J Med 2014;370:33-40.
- Feldman D, Pamboukian SV, Teuteberg JJ, et al. The 2013 International Society for Heart and Lung Transplantation guidelines for mechanical circulatory support: Executive summary. J Heart Lung Transplant 2013;32:157-187.
- Sareyyupoglu B, Boilson BA, Durham LA, et al. B-type natriuretic peptide levels and continuous-flow left ventricular assist devices. ASAIO J 2010;56:527-531.
- Hellman Y, Malik AS, Lin H, et al. B-type natriuretic peptide-guided therapy and length of hospital stay post left ventricular assist device implantation. ASAIO J 2015;61:156-160.
- Neal JR, Quintana E, Pike RB, et al. Using daily plasma-free hemoglobin levels for diagnosis of critical pump thrombus in patients undergoing ECMO or VAD support. J Extra Corpor Technol 2015;47:
103-108. - Auerbach SR, Richmond ME, Schumacher KR, et al. Infectious complications of ventricular assist device use in children in the United States: Data from the Pediatric Interagency Registry for Mechanical Circulatory Support (PediMACS). J Heart Lung Transplant 2018;37:46-53.
- Chetan D, Buchholz H, Bauman M, et al. Successful treatment of pediatric ventricular assist device thrombosis. ASAIO J 2018;64:e28-e32.
- Jennings DL, Jacob M, Chopra A, et al. Safety of anticoagulation reversal in patients supported with continuous-flow left ventricular assist devices. ASAIO J 2014;60:381-384.
- Rimsans J, Levesque A, Lyons E, et al. Four factor prothrombin concentrate for warfarin reversal in patients with left ventricular assist devices. J Thromb Thrombolysis 2018;46:180-185.
- Perberdy MA, Gluck JA, Ornato JP, et al. Cardiopulmonary resuscitation in adults and children with mechanical circulatory support: A scientific statement from the American Heart Association. Circulation 2017;135:e1115-e1134.
Fortunately, pediatric heart failure is a rare occurrence, but early diagnosis, aggressive management, and timely transfer to a facility capable of advanced cardiac support are essential to optimize the outcome of each child. The authors review the early recognition of a child in heart failure and also discuss an approach to troubleshooting and recognizing complications associated with a ventricular assist device.
Subscribe Now for Access
You have reached your article limit for the month. We hope you found our articles both enjoyable and insightful. For information on new subscriptions, product trials, alternative billing arrangements or group and site discounts please call 800-688-2421. We look forward to having you as a long-term member of the Relias Media community.
