Pediatric Asthma Management in the Emergency Department
July 1, 2020
AUTHORS
Jaymin Modi, DO, Emergency Medicine Resident, Department of Emergency Medicine, University of North Carolina, Chapel Hill
Daniel Migliaccio, MD, Clinical Assistant Professor, Department of Emergency Medicine, University of North Carolina, Chapel Hill
PEER REVIEWER
Aaron Leetch, MD, FACEP, Associate Professor, Director Pediatric Emergency Medicine Residency, University of Arizona, Tucson
EXECUTIVE SUMMARY
• Asthma is a complex and dynamic disease revolving around the concepts of underlying inflammation, airway hyperresponsiveness, and airway obstruction.
• Important aspects of the history includes onset of symptoms, severity compared to prior exacerbations, intubations, intensive care admissions, and complicating cardiac or pulmonary illnesses. Social history factors associated with increased mortality in asthma include low socioeconomic status or inner-city residence and major psychosocial problems.
• When wheezing is present within the first few months without signs of viral illness, shows no response to therapy, and is persistent, the provider must consider structural causes like tracheobronchial malacia and vascular rings.
• Implementing a clinical pathway for pediatric ED asthma management leads to improved adherence to evidence-based practices and fewer hospital admissions.
• The two major environmental factors involved in the development, persistence, and severity of asthma are airborne pathogens and viral respiratory pathogens. Some evidence suggests that respiratory virus infections in early life are more frequently associated with asthma development. Fifty percent of infants with respiratory infections caused by respiratory syncytial virus (RSV) during the first 12 months of life developed persistent asthma by school age.
• Human rhinovirus and RSV are the most common viral illnesses associated with wheezing early in life and the development of asthma and exacerbations.
• It is important to consider that although only 20% of foreign bodies are radiopaque, abnormal findings are present in 40% to 80% of cases, with unilateral hyperinflation from obstructive emphysema, mediastinal shift, atelectasis, or pneumonia.
• For children with moderate to severe asthma, ipratropium reduces the rate of hospitalization when given with second and third albuterol doses. Corticosteroid use in acute asthma is associated with reduced inflammation, decreased mucus production, and enhanced beta agonist activity. Use of corticosteroids within one hour of arrival to an ED with acute asthma exacerbations reduces the need for hospital admission.
• Suspicion for anaphylaxis should increase when there is a slow response to bronchodilator therapy after rapid onset of symptoms, particularly wheezing with urticaria and hypotension, which should lead to a trial of epinephrine for suspected anaphylaxis.
• In severe asthma exacerbations not responsive to initial bronchodilator or steroid therapy and where alternative diagnoses have been discussed, the next tier of therapy is indicated. This includes consideration of terbutaline, epinephrine, and intravenous magnesium.
Asthma is the most common chronic disease of childhood. Children with asthma frequently present in the acute care setting with disease ranging from mild to severe. Accurately assessing children with asthma and providing escalating care as needed improves outcome. The authors provide a current review of asthma and evidence-based care.
— Ann M. Dietrich, MD, FAAP, FACEP
Asthma is a chronic disease diagnosed based on patient symptoms including wheezing, shortness of breath, and coughing. The diagnosis is supported by evidence of airway narrowing on a spirometer, which is reversible with bronchodilator therapy. Common triggers include infection or allergens.1
An exacerbation is an accentuation of existing inflammatory processes and a loss of disease control.2 Among all pediatric patients, acute asthma exacerbations are one of the most common reasons for presentation to the emergency department (ED) and hospitalization.3
Epidemiology and Etiology
In the United States alone, 60% of children with diagnosed asthma will have at least one acute exacerbation per year, and up to one in five of them will require a visit to the ED annually.3-5
According to the Global Initiative for Asthma guidelines (GINA), 300 million individuals have asthma worldwide, and it is the most common chronic disease of childhood. It affects up to 18% of all pediatric patients and is projected to affect an additional 100 million persons by 2025.6 Overall, asthma is estimated to account for 1.1% of disability-adjusted life years (DALYs) per 100,000 population for all causes.7
A combination of host and environmental factors continues to increase the prevalence and mortality of asthma in children, especially in low-middle income countries. Host factors such as atopy and eczema, along with environmental factors such as tobacco smoke exposure and air pollution, have a direct correlation with rising asthma prevalence.6,7
The two major environmental factors involved in the development, persistence, and severity of asthma are airborne pathogens and viral respiratory pathogens.8
Some evidence suggests that respiratory virus infections in early life are more frequently associated with asthma development. Fifty percent of infants with respiratory infections caused by respiratory syncytial virus (RSV) during the first 12 months of life developed persistent asthma by school age.9 It remains unclear whether bronchiolitis early in life causes future asthma or simply serves as a marker for susceptibility of pediatric asthma.10
Asthma severity often is divided into four groups: intermittent, mild persistent, moderate persistent, and severe persistent. According to the Expert Panel Report 3 (EPR-3), asthma severity is determined by impairment and risk.11 Impairment is based on the impact on day-to-day activities, including daytime symptoms, nighttime awakenings, frequency of short-acting beta agonist use for relief, interference with normal activities, and spirometry. Risk is based on the number of asthma exacerbations requiring oral systemic steroids per year.
As detailed in Table 1 (available at https://bit.ly/2MNfzKd), asthma is considered persistent once the patient is averaging short-acting inhaler use more than two days per week or has minor limitation in normal activities. At that point, the patient should be prescribed a daily controller medication, preferably a low-dose inhaled corticosteroid in addition to a short-acting beta agonist (see Table 2, available at https://bit.ly/2UqJNaf).
According to the Centers for Disease Control and Prevention (CDC), 60% of children with asthma have persistent asthma, compared to 40% who have intermittent asthma.12
Pathophysiology
Asthma is a complex and dynamic disease revolving around the concepts of underlying inflammation, airway hyperresponsiveness, and airway obstruction. It causes various symptoms, including wheezing, shortness of breath, coughing, and chest tightness.8
Airway obstruction often results from the constriction of bronchial smooth muscle in response to stimuli such as allergens irritants. The allergen can trigger an immunoglobulin E- (IgE) or non-IgE-dependent release of histamine, leukotrienes, prostaglandins, and tryptase from mast cells that directly trigger airway constriction.
Persistent inflammation can produce edema and hypertrophy of smooth muscle in the airway, leading to airway remodeling that is only partially reversible. Inflammation also can contribute to airway hyperresponsiveness or an exaggerated bronchoconstrictor response to stimuli.
In patients with significant remodeling, episodes of airway hyperresponsiveness lead to increased ED visits for exacerbations that cannot be controlled with home rescue therapy. Patients often present to the ED hypoxic, secondary to ventilation perfusion mismatch from reflex hypoxic vasoconstriction. This reflex hypoxic vasoconstriction is in addition to the ventilation perfusion mismatch caused by mucus plugging and bronchospasm. Many potential contributors to inflammation can trigger acute asthma symptoms, including various interleukins, neutrophils, mast cells, and eosinophils. (See Figure 1.) Treatment is often unique to each patient and is targeted toward reducing inflammation and reducing airway obstruction, hyperresponsiveness, and long-term remodeling.
Figure 1. Factors Limiting Airflow in Acute and Persistent Asthma |
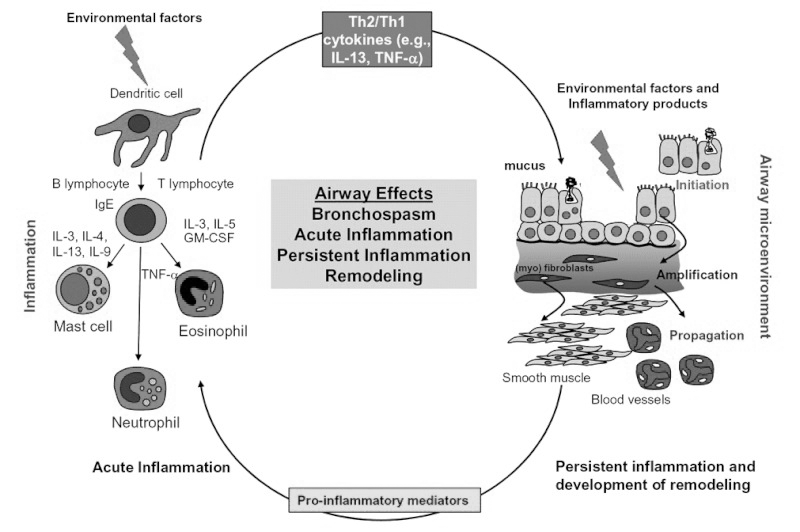 Key: GM-CSF, granulocyte-macrophage, colony-stimulating factor; IgE, immunoglobulin E; IL-3, interleukin 3 (and similar); TNF-α, tumor necrosis factor-alpha. Adapted and reprinted from: Holgate ST, Polsa R. The mechanisms, diagnosis, and management of severe asthma in adults. Lancet 2006;368:780-793, with permission from Elsevier. |
Differential Diagnoses
In addition to asthma, other causes of small airway obstruction presenting with similar symptoms include viral bronchiolitis, bronchopulmonary dysplasia, cystic fibrosis, and congenital heart diseases. Pediatric patients with and without prior diagnoses of asthma often present to the ED with symptoms of wheezing, shortness of breath, coughing, and chest tightness.
The differential for such symptoms is broad and often challenging in younger patients, who are unable to provide a history. Viral bronchiolitis is the most common lower respiratory tract infection in infants, often presenting with rhinitis, nasal flaring, fever, and tachypnea in addition to bronchospasm and cough.13 It is the most common wheezing illness in infancy and must be considered as a primary diagnosis until at least age 2 years.14
Human rhinovirus (RV) is detected most often in the spring and fall, compared to RSV, which is detected most often in the winter months. Unlike in asthma, bronchodilators and steroids do not affect the rate or duration of hospitalization in patients with bronchiolitis and no history of established asthma.
Treatment of RSV is primarily supportive therapy (i.e., aggressive nasal suctioning) and supplemental oxygen as needed. Early childhood viral infections can lead to pathophysiological changes, making children more prone to asthma and subsequent exacerbations. There are several theories as to the mechanism, including changes to the airway microbiome.15
Upper airway obstruction, such as foreign body aspiration, tracheobronchial malacia, laryngomalacia, vocal cord dysfunction, and vascular rings, are also possible diagnoses, particularly when the patient presents with stridor instead of wheezing. Congenital laryngomalacia is the most common cause of stridor in infants.16 Dyspnea on exertion, in the absence of other symptoms of asthma or minimal response to albuterol, is most likely the result of a condition other than asthma.17
For these reasons, history, respiratory sounds, spirometry, and blood gas may help avoid improper diagnosis of asthma. When wheezing is present within the first few months without signs of viral illness, shows no response to therapy, and is persistent, the provider must consider structural causes like tracheobronchial malacia and vascular rings.18 Tracheobronchial malacia is the softening or weakening of the tracheobronchial tree, causing the airway to collapse or narrow from either intrinsic or primary conditions, such as extreme prematurity, or external compression from vascular structures, such as a double aortic arch.19 A vascular ring is a congenital encasement of the trachea and esophagus circumferentially by vascular structures, often a common dorsal aorta caused by the unification of persistent embryonic arches.20
Anaphylaxis must always be considered, particularly in patients presenting with impending respiratory failure from suspected acute asphyxic asthma. Patients who are having an anaphylactic reaction but presenting primarily with respiratory symptoms often are misdiagnosed with acute asthma.21 Suspicion should increase when there is a slow response to bronchodilator therapy after rapid onset of symptoms, particularly wheezing with urticaria and hypotension, which should lead to a trial of epinephrine for suspected anaphylaxis.21 Failure to give epinephrine in a timely manner is the most important factor contributing to mortality in patients presenting with systemic anaphylaxis.22
Often, the triggers for anaphylaxis are foods and medications that can be determined through allergy testing, and such patients are appropriately prescribed self-administering epinephrine devices that have an onset of action within 10 minutes.21,23
Regardless, some overlap remains between anaphylaxis and severe acute asthma, particularly in the setting of allergens that have yet to be identified, leading to underdiagnosis and undertreatment of anaphylaxis in favor of suspected asthma.21
Clinical Features
Pediatric asthma exacerbations can have a wide range of presentations, and it is imperative to recognize key clinical features. To assist with triaging of suspected asthma exacerbations, the Children’s Hospital of Philadelphia (CHOP) created an ED asthma pathway. To view the pathway, visit https://www.chop.edu/clinical-pathway/asthma-emergent-care-clinical-pathway. This pathway incorporates the Pediatric Asthma Severity Score (PASS), which triages patients in descending acuity from level 1 to 5.
Level 1 patients are in critical condition with severe wheezing or stridor with severe tachypnea, retractions, grunting respirations, decreased tone, bradypnea, lethargy, absent breath sounds, and agonal respiration with pulse oximetry less than 90%. According to the EPR-3, patients also may have absence of wheezing, and paradoxical thoracoabdominal movement.
Level 2 patients are considered acute and will have decreased aeration with moderate tachypnea, still often with stridor at rest. According to the CHOP pathway, any asthmatic patient with a history of intubation presenting at triage with suspected asthma exacerbation should be at least a level 2 triage initially.
Level 3 and 4 patients are considered urgent. Level 3 patients have wheezing or stridor with only a mild increase in work of breathing with mild retractions and tachypnea. In comparison, level 4 patients may have wheezing but only minimal work of breathing and an initial pulse oximetry greater than 95%.
Level 5 patients are considered non-urgent. Two or more hospitalizations in the past year or three or more ED visits for asthma in the past year are associated with increased asthma mortality.8
Other important aspects of the history includes onset of symptoms, severity compared to prior exacerbations, intubations, intensive care admissions, and complicating cardiac or pulmonary illnesses. Social history factors associated with increased mortality in asthma include low socioeconomic status or inner-city residence and major psychosocial problems.
The physical examination entails evaluation of vital signs and pulse oximetry, hydration status, alertness level, presence of cyanosis, and observation for immediate complications, such as pneumothorax and upper airway obstruction.24 It is essential to consider additional diagnoses, such as a foreign body, before proceeding to additional asthma-targeted therapy, particularly in pediatric patients presenting acutely ill who do not respond to initial therapy.
Only 50% to 70% of children present to the ED within 24 hours of aspiration.25 In addition to questioning caretakers on a recent choking event, on physical exam, assess for decreased breath sounds on one side, usually the right side given the angle of bronchi. Also monitor for lack of improvement with asthma treatment.
It is also important to consider that although only 20% of foreign bodies are radiopaque, abnormal findings are present in 40% to 80% of cases, with unilateral hyperinflation from obstructive emphysema, mediastinal shift, atelectasis, or pneumonia.26 If suspicion exists for a foreign body, perform a two-view X-ray. (See Figure 2.)
Figure 2. Chest X-ray with Bronchial Foreign Body |
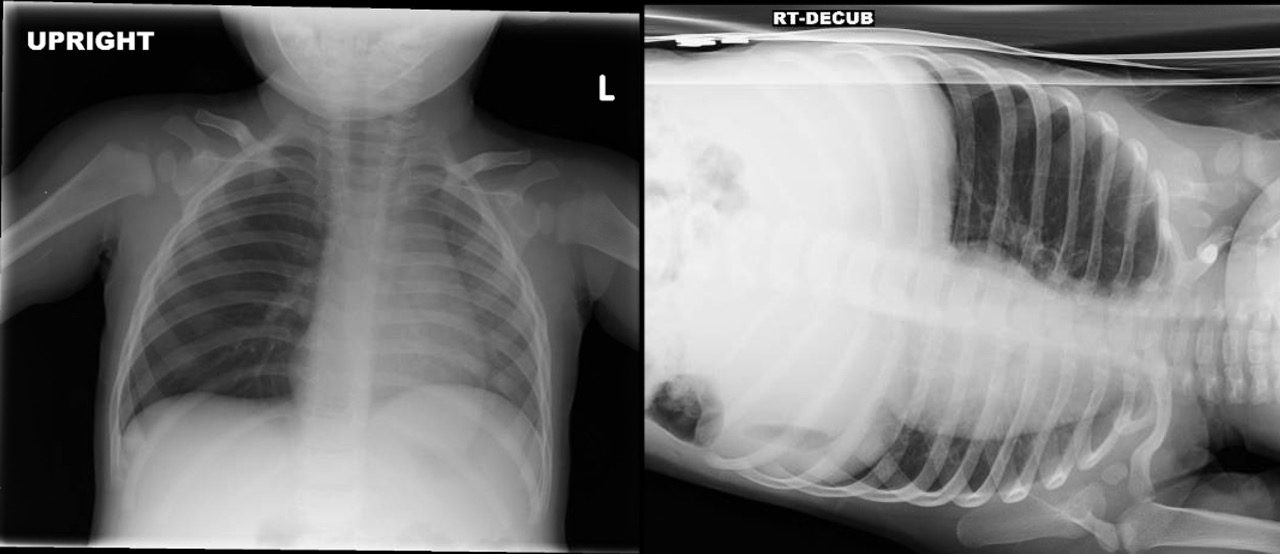 Source: Photo by Aaron Leetch. Pediatric Emergency Medicine Reports, January 2016. |
Clinical Scoring Systems
There are many different scoring systems used for asthma exacerbations, including PASS, Respiratory Score (RS), and Pediatric Asthma Score (PAS). (See Table 3.)
Table 3. Strengths and Weaknesses of the PASS, RS, and PAS |
|||
|
Scoring System |
Overview |
Advantages |
Disadvantages |
|
PASS* |
Three categories scored from 0-2 |
|
|
|
RS** |
Four categories scored from 0-3, stratified by age |
|
|
|
PAS*** |
Five categories scored from 0-3 |
|
|
|
*Pediatric Asthma Severity Score **Respiratory Score ***Pediatric Asthma Score |
|||
The PASS assesses for three categories of clinical findings, including wheezing, work of breathing, and prolongation of expiration. PASS uses a score of 0 for mild, 1 for moderate, and 2 for severe findings in each category.
For example, mild wheezing would be given a score of 0, moderate wheezing would be given a score of 1, and severe wheezing — or absent due to poor air movement — would be given a score of 2. If the patient has a score of 2 or more, consider inpatient admission. Scores less than 2 suggest outpatient treatment is reasonable. The scoring system has been validated for use in pediatric patients from ages 1 to 18 years, with scores assigned on initial assessment and after ED treatment.
The benefits of the PASS system include its ease of use, minimal cost, and reliability among providers from nurses to respiratory therapists to physicians. In addition, the scores before and after treatment were consistent with other measures of asthma severity, such as peak expiratory flow rate (PEFR), and were responsive to patient status during the course of ED treatment. Unlike several other scoring systems, the PASS has been tested in a large patient population with a broad age range and exacerbation severity and no exclusions for severe exacerbation or inability to perform PEFR.
The biggest limitation of the PASS is that it is based on only three categories, which may cause hesitation in some clinicians.27 Alternatively, the RS provides a more detailed scoring system with four categories: respiratory rate, retractions, dyspnea, and auscultation. The RS is scored from 0 to 3 points, with a maximum total of 12 points. Both respiratory rate and dyspnea are subdivided into age groups, while retractions and auscultation are applied similarly to all patients. Based on the limited data used to study this scoring system, the PASS has been shown to decrease ED length of stay. However, there is no shown decrease in inpatient or intensive care admissions or inpatient length of stay.28
The acute asthma pathway developed at Seattle Children’s Hospital involves taking an RS at triage and every hour afterward. For example, an RS of less than 6 at triage involves the patient receiving steroid dose and albuterol given via metered dosed inhaler (MDI). Meanwhile, a score of 6 or greater results in the patient receiving a steroid dose and albuterol continuous nebulization therapy, with ipratropium for patients older than 2 years of age. Similarly, there are additional decision-making pathways following the RS score at hour 2, divided into an RS of less than 5, an RS of 5-8, and an RS of more than 8.29 Compared to the PASS, the RS provides more specific management recommendations because it originally was intended to guide inpatient management of asthma exacerbations. Similarly, the PAS also was developed originally to guide inpatient management of asthma exacerbations, but it has been extended to the ED. (See Table 4.) The PAS has five categories: respiratory rate, oxygen requirement, auscultation, retractions, and dyspnea, with a maximum score of 15.
Table 4. Determining the Severity Level of Asthma with the Pediatric Asthma Score |
|||
|
Examination Component |
1 |
2 |
3 |
|
Respiratory rate |
|||
|
1-4 years old 4-6 years old 6-12 years old More than 12 years old |
34 or less 30 or less 26 or less 23 or less |
35-39 31-35 27-30 24-27 |
40 or more 36 or more 31 or more 28 or more |
|
O2 requirement |
More than 95% on room air |
90% to 95% on room air |
Less than 90% on room air or any oxygen |
|
Retractions |
None or intercostal |
Intercostal and substernal |
Intercostal, substernal, and supraclavicular |
|
Work of breathing |
Speaks in sentences, coos, and babbles |
Speaks in partial sentences, short cry |
Speaks in single words/short phrases, grunting |
|
Auscultation |
Normal breath sounds to end-expiratory wheezes only |
Expiratory wheezing |
Inspiratory and expiratory wheezing to diminished breath sounds |
|
Reprinted with permission: Nievas IF, Anand KJ. Severe acute asthma exacerbation in children: A stepwise approach for escalating therapy in a pediatric intensive care unit. J Pediatr Pharmacol Ther 2013;18:88-104. |
|||
Scores of 5-7 are considered a mild exacerbation, scores of 8-11 are considered moderate, and scores of 12-15 are considered severe. Although primary data for the PAS were based on a small sample size and retrospective studies, the use of the PAS was found to decrease cost and to increase the likelihood of oral steroid dose vs. intravenous (IV) steroids, asthma education, follow-up, and a controller prescription upon discharge.30
Asthma scoring systems have been found to be useful in guiding management and disposition, but they are not meant to replace clinical judgment. For example, it is possible for a patient to be hypoxemic with minimal distress and thus have a low RS. In this situation, clinical judgment must have a significant role. For this reason, several institutions, such as CHOP, include a section for history and assessment in their ED pathway for asthma exacerbations. This includes a brief history and examination, as previously discussed.
Diagnostic Studies
The role of chest X-rays for patients with respiratory distress consistent with asthma is limited, unless a foreign body is suspected.31 A retrospective study analyzing more than 1,500 asthma visits over a two-year period found that the physician obtained chest X-rays in nearly 400 visits. The most associated findings in the chest X-ray patient group were: fever greater than 38.3°C, symptoms longer than two days, age younger than 5 years, respiratory rate more than 40 breaths per minute, or pulmonary rales. Of the chest X-rays ordered, 10% had an infiltrate and 5% had atelectasis.
Only ages younger than 5 years and pulmonary rales were associated with abnormal radiographs. No significant difference was found in the X-rays of patients treated as outpatients and those requiring hospitalization. A more recent retrospective study on children with acute asthma exacerbations who received a chest X-ray in a pediatric ED showed only previous antibiotic administration in the past seven days had a significant relationship with both pneumonia found on chest X-rays and antibiotic administration in the ED.32 The data, although retrospective in nature, suggest that chest X-ray utility is limited in terms of disposition and management of suspected asthma exacerbations presenting to the ED.
As the role of chest X-ray in acute asthma exacerbation diminishes, research on the use of ultrasound continues to evolve. Although lung ultrasound patterns in children with asthma are yet to be determined, the utility of point-of-care ultrasound is the ability to identify alternative diagnoses and, thus, guide management. More specifically, point-of-care ultrasound can accurately detect pneumothorax, consolidative pneumonia, and focal atelectasis, offering a fast, cost-effective option that helps guide management.33
In a 2017 cross-sectional study, Dankoff et al attempted to categorize lung ultrasound findings in children with asthma presenting to the ED with respiratory distress.33 The study sonographer attended a two-day ultrasound course to learn a six-zone linear transducer protocol examining bilateral anterior, mid-axillary, and posterior chest zones. Children ages 2-17 years who were determined to have at least a moderate asthma exacerbation by a nurse-driven, physician-blinded protocol were evaluated after receiving steroid and beta agonist treatment.
A positive ultrasound was defined as multiple B lines, consolidation, or pleural abnormalities. A negative lung ultrasound was defined as the presence of A lines without other findings. 90% of all study patients were diagnosed with a pure asthma exacerbation by the physician, 41% of whom had positive ultrasounds as determined by the sonographer. On the contrary, 10% were diagnosed with both pneumonia and asthma by the physician, and 85% of those patients had positive ultrasounds. Overall, a positive lung ultrasound correlated to prolonged length of stay in the ED, higher admission rate, and additional therapies, such as oxygen and magnesium. In contrast to chest X-ray, early evidence shows there may be a role for point-of-care ultrasound in patient disposition and earlier goal-directed treatment.
Like ultrasound and chest X-ray, there may be a role for viral panel testing in pediatric patients presenting to the ED for suspected acute asthma exacerbation, particularly those patients who are likely to be admitted. RV and RSV are the most common viral illnesses associated with wheezing early in life and the development of asthma and exacerbations. Up to 85% of asthma exacerbations in children are linked to viral infections and contribute to the severity of exacerbations.15,34 However, there remains no strong objective clinical data supporting viral polymerase chain reaction (PCR) testing in non-hospitalized pediatric asthma exacerbations.35
Chang et al examined children 2-15 years of age presenting to the ED for acute asthma exacerbation but who were not hospitalized and found no clinically significant association between the presence of acute viral illness via PCR and quality of life (QOL) score on presentation to the ED, day 7, or day 14 of illness.35
Viruses assessed in this study included influenza, RSV, RV, adenovirus, parainfluenza, coronavirus subtypes, as well as chlamydia and mycoplasma. Respiratory viruses are present in most patients hospitalized for acute asthma and life-threatening asthma and may contribute to mortality in these patients.36
According to an analysis of a prospective cohort of nearly 1,000 children presenting to the ED with moderate to severe asthma exacerbations, detection of a respiratory pathogen was not associated with higher severity on presentation to the ED, but it was associated with increased treatment failure risk, defined as hospital admission, ED stay of more than eight hours, or relapse presentation. Parainfluenza was associated with the highest treatment failure risk at nearly 35%.37
Viral testing in hospitalized pediatric patients also assists in management through respiratory precautions, contributing to the safety of surrounding patients and family members. For this reason, hospitalized pediatric patients with acute asthma exacerbations may benefit from viral testing.
Approach to Management
Implementing a clinical pathway for pediatric ED asthma management leads to improved adherence to evidence-based practices and fewer hospital admissions.38 Studies from Canada, South America, and the United Kingdom have shown that pediatric asthma exacerbation pathways not only decrease hospitalization rates, but also decrease prescribing errors and return visits.
However, in the United States, pediatric asthma management in the ED is variable, with pathway implementation often limited by a lack of follow-up options and a fee-for-service system.39
It should be acknowledged that there are many different asthma pathways available, and there is no evidence to support the superiority of one pathway over another.
The benefits of implementing an asthma pathway come from the standardization and expedition of care, including timely administration of steroids and bronchodilators, which are known to decrease admission rates.
Every 30-minute delay in ED steroid administration past one hour is associated with a 20% increase in the odds of admission for pediatric asthma exacerbation.40 In this review, the CHOP Pediatric ED asthma pathway is emphasized. CHOP has a robust clinical pathways program led by multidisciplinary care teams.
CHOP has more than 100 pathways, with four such pathways on different phases of asthma care alone. Furthermore, the CHOP pathway incorporates and highlights the PASS system, which promotes ease of use and prompt triaging, and is supported by randomized, controlled trial data in the ED.
When a patient arrives in the triage area of the ED with a suspected acute asthma exacerbation, the initial step should focus on rapid assessment and assigning a level of acuity from 1 to 5, followed by a brief assessment of pertinent history and examination.
One of the most important components of the initial screening is evaluating and reversing hypoxemia. One set of criteria cannot define severity accurately given the wide spectrum of disease.
Pulmonary function tests are objective, but they require both appropriate effort by the patient and must be interpreted with consideration of baseline values, if available, or predicted value based on height. This highlights pulse oximetry as an invaluable component of the initial assessment because it provides objective and real-time assessment of overall asthma severity. Oxygen saturation should be obtained immediately, and if it remains below 90%, supplemental oxygen should be given.41 There is no evidence of oxygen suppressing respiratory drive in children with severe asthma.42
Several options are available for noninvasive oxygen therapy, including nasal cannula, high flow nasal cannula (HFNC), simple masks, nonrebreather masks, and continuous and bilevel positive airway pressure (CPAP and BIPAP). (See Table 5.) Simple masks can provide up to 10 L of flow per minute and a fraction of inspired oxygen (FiO2) up to 60%. Nonrebreather masks deliver up to 15 L of flow per minute and an FiO2 close to 100%.43 Of note, at lower flow rates, a nonrebreather mask could lead to an ineffective washout of exhaled carbon dioxide and the potential to rebreathe exhaled gases.44
Table 5. An Overview of Oxygen Delivery Methods |
|||||
|
Oxygen Delivery Method |
Oxygen Flow Rate |
FiO2 |
Design |
Advantages |
Limitations |
|
Nasal cannula |
|
40% |
Nasal prongs |
|
|
|
High flow nasal cannula |
|
100% |
Nasal Prongs, heated and humidified |
|
|
|
Simple mask |
|
60% |
Face mask |
|
|
|
Nonrebreather mask |
|
100% |
Mask with one-way valve, reservoir bag |
|
|
|
Continuous positive airway pressure |
|
100% |
Face or nasal mask |
|
|
|
Bilevel positive airway pressure |
|
100% |
Face or nasal mask |
|
|
A nasal cannula can provide an FiO2 up to 40% with 6 L of flow per minute. Although the definition of HFNC varies, flow rates of more than 6 L/minute up to 30 L/minute generally are considered high flow in children, whereas flow rates of 2 L/minute adjusted up to 2 L/kg/minute are considered high flow in infants, with FiO2 approaching 100%.45-47 In addition to increased flow rates and potential FiO2 in HFNC, the mixture of air is heated and humidified, which decreases mouth dryness and resistance in the nasal mucosa, improving patient tolerance and oxygen flow rates to distal airways.45 Furthermore, HFNC can create a positive end-
expiratory pressure (PEEP) up to
6 cm H2O during expiration, similar to CPAP.48
Several potential drawbacks of HFNC exist. In theory, lack of precise measurement of pressures generated in the airways could, contribute to pneumothorax risk.45
In addition, although nebulized albuterol can be given in line with an HFNC circuit, drug delivery decreases with an increase in flow rates, an issue that can be avoided altogether with CPAP and BIPAP.49 CPAP and BIPAP are administered using conventional ventilators and create positive pressure through a face mask or nasal mask that distends the distal airways to improve lung oxygenation and volumes while decreasing work of breathing.50 CPAP delivers one constant pressure throughout the respiratory cycle, typically starting at 4-6 cm H2O, and titrates based on work of breathing.
BIPAP requires two pressure settings, with inspiratory positive airway pressure (IPAP) typically starting at
10-12 cm H20 and expiratory positive airway pressure (EPAP) typically starting at 4-6 cm H2O. Heliox is an option for severe exacerbations. With narrowing of the airways in acute asthma, the velocity of gas in the airway increases and leads to turbulent flow, with increased airway resistance and leading to decreased deposition of inhaled oxygen and medication to distal alveoli.51
Heliox is oxygen mixed with helium, which is a gas that is much less dense than air, leading to increased distal laminar flow. A recent meta-analysis has shown an improvement in asthma severity scores following two hours of heliox use in pediatric patients with severe exacerbations, particularly with a mixture of 70% helium and 30% oxygen.4
Following the initial rapid assessment and assignment of the PASS to determine severity level, treatment should be initiated accordingly. If the patient is determined to have be level 4, with mild symptoms and a PASS of 0 or 1, consider a dexamethasone tablet, albuterol MDI (two to four puffs), and likely discharge them.
If the patient is level 3 with a PASS of less than 3, give a dexamethasone tablet and albuterol MDI every 20 minutes for three doses total and reassess after an hour without any treatment for symptom recurrence. If symptoms can be classified as mild one hour post-treatment, consider discharging the patient.
Patients with severe exacerbations are likely to be taken to the resuscitation bay immediately from triage, then given a steroid dose and albuterol-
ipratropium combination nebulizer treatment. Beta agonists such as albuterol cause bronchodilation via beta-2 receptor-activated bronchial smooth muscle relaxation.52 Ipratropium is an atropine derivative that aids in bronchodilation by inhibiting cholinergic-mediated bronchospasm and is an important agent in treating moderate-to-severe asthma in the ED. Ipratropium reduces the rate of hospitalization when given with second and third albuterol doses, presuming a steroid dose was given on arrival.53-55
Corticosteroid use in acute asthma is associated with reduced inflammation, decreased mucus production, and enhanced beta agonist activity.56 Use of corticosteroids within one hour of arrival to an ED with acute asthma exacerbations reduces the need for hospital admission, with a number needed to treat of 8.57 More specifically, as it relates to asthma pathways, triage nurse initiation of oral corticosteroids in children with moderate-to-severe asthma exacerbations, prior to physician assessment, has been associated with earlier clinical improvement, discharge, and reduced admission rates.58
According to the CHOP pathway, steroid options are oral or IV prednisone (2 mg/kg up to a maximum of
60 mg) or dexamethasone tablet in a mild-to-moderate flare, stratified by weight, and often crushed in juice or syrup. More specifically, give a 4 mg tablet for children weighing 5-8 kg, a
6 mg tablet for children weighing
8-12 kg, and an 8 mg tablet for children weighing more than 12 kg.
A single dose of intramuscular dexamethasone (0.6 mg/kg) resulted in no clinically significant difference in outcomes compared to a five-day course of oral prednisone for treatment of moderate acute asthma exacerbations discharged from the ED.59 Subsequent studies showed no significant difference between intramuscular and oral dexamethasone.56 In addition to a shorter course of therapy, decreased cost, and an implied increase in compliance, patients receiving dexamethasone had significantly decreased instances of vomiting in the ED or at home following discharge compared to those who received prednisone.60
In severe asthma exacerbations not responsive to initial bronchodilator or steroid therapy, and where alternative diagnoses have been discussed, the next tier of therapy is indicated. This includes consideration of terbutaline, epinephrine, and IV magnesium.
In severe asthma, defined as a PASS score of more than 3, consider repeating albuterol nebulized treatments and IV magnesium. Intravenous magnesium has been shown to improve pulmonary function in children with acute asthma when given at 50 mg/kg up to a maximum of 1,500 mg over 60 minutes.36
The CHOP pathway extends the maximum dose to 2,000 mg. IV magnesium also has been shown to reduce the odds of hospital admission for acute pediatric asthma with no significant side effects, according to recent reviews.38,61
The mechanism of action of magnesium is theorized to be blockage of N-methyl-D-aspartate (NMDA) receptor-gated calcium channels, resulting in muscle relaxation and bronchodilation.62-64
Although concerns remain regarding magnesium-induced muscle weakness and pervasive vasodilatation leading to respiratory failure and hypotension, this is not supported with evidence. While earlier studies showed minimal or no adverse effects, fear of potential side effects remains widespread.65
To encourage use of magnesium, the CHOP pathway for magnesium administration recommends a
20 mL/kg bolus of normal saline given in parallel, with vital signs checked every 15 minutes and ED observation for one hour prior to inpatient floor transfer. The results of a randomized, controlled trial with 100 patients in India showed early IV magnesium sulfate was superior to terbutaline, determined by a modified asthma clinical severity score.66
However, the CHOP asthma pathway suggests early terbutaline for severe asthma exacerbations out of a concern for pending respiratory failure. The initial dosing is 10 mcg/kg or a 250 mcg maximum bolus, given subcutaneously before IV placement, or 2-10 mcg/kg IV with a maximum of 750 mcg.
An IV beta-2 agonist should be considered in patients who do not improve on continuous albuterol. Terbutaline is considered the drug of choice in the United States.67 Of note, when terbutaline is given subcutaneously, it loses beta selectivity and has no perceived advantage over epinephrine.68
Previous studies showed that, when given in dose of 0.01 mg/kg with a maximum of 0.25 mg, both epinephrine and terbutaline resulted in bronchodilation within five minutes, which was sustained for four hours, and no clinically significant side effects were noted.69 Potential side effects of beta agonists include tachycardia, diastolic hypotension, arrythmias, QT prolongation, and hypokalemia.70,71
For patients with severe asthma who fail to improve or deteriorate despite optimization of conventional therapy, noninvasive positive pressure ventilation has been shown to improve clinical exam and gas exchange, often in patients as young as 1 year of age.72
The combination of air trapping, bronchospasm, and airway obstruction leads to severe lung hyperinflation and increased positive intrapleural pressure. This positive pressure decreases passive air movement and leads to tachypnea, shortened inspiration, and worsening hyperinflation.73
Noninvasive ventilation methods, including HFNC, CPAP, and BIPAP, oppose intrinsic PEEP and recruit previously collapsed alveoli to increase total lung capacity toward baseline.74 BIPAP also may increase delivery of nebulized albuterol to areas of poor ventilation.75 With an increased inspiratory positive airway pressure, a decreased expiratory positive airway pressure, and a prolonged inspiration-to-expiration ratio, there are fewer intubations, pediatric intensive care unit admissions, and overall improvements in PASS scores.74
Although large randomized studies are lacking, many small studies and case reports suggest that a combination of ketamine and BIPAP may be beneficial in severe asthma exacerbations and may help avoid mechanical ventilation.76,77 Ketamine given at a loading dose of 1 mg/kg followed by a continuous infusion for one to two hours has been associated with improvement in asthma scores, oxygen saturations, and mean peak airway pressures.77
Ketamine is a phencyclidine derivative that blocks NMDA receptor-induced bronchospasm through smooth muscle relaxation and depression of nitric oxide levels and inflammatory markers.78,79 The known dissociative effects of ketamine without suppression of respiratory drive increases chest wall compliance and overall synchrony with noninvasive or invasive ventilation.77,80 Ketamine also has favorable pharmacologic properties, including an onset of action within 60 seconds and a single bolus duration of action of up to 15 minutes.81
The most common side effects of ketamine often are treatable or preventable. Increased airway secretions and hallucinations can be addressed with atropine and benzodiazepines, respectively.77 Although infrequent, laryngospasm, a transient rise in intracranial pressure, and apnea can occur with higher doses or rapid administration.81,82
If laryngospasm occurs, proceed with a chin lift, jaw thrust technique followed by CPAP and assessment of air entry and bag movement. If the patient remains in complete laryngospasm, administer propofol to deepen anesthesia for subsequent endotracheal intubation.83
The decision to progress from pharmacologic and noninvasive therapy to intubation and mechanical ventilation is based on clinical judgment. Studies support a wide variability in practice, but the decision should not be delayed once deemed necessary.84,85 Signs of hypercarbic and hypoxic respiratory failure with altered mental status and metabolic acidosis from anaerobic metabolism despite optimization are signs that intubation and mechanical ventilation are necessary.86 Permissive hypercapnia, increase in expiratory time, and ventilator synchrony are the mainstay of mechanical ventilation.41 Neither a pressure- nor volume-targeted mode of ventilation has been proven to be superior, but the goal is to limit barotrauma and hyperinflation while providing adequate gas exchange.87
Hyperinflation occurs as a result of breath stacking, or unintended high tidal volumes from incomplete exhalation between inspiratory cycles. Hyperinflation is best determined by end inspiratory plateau pressure and PEEP. The American College of Chest Physicians recommends maintaining end inspiratory plateau pressure less than 35 cm H2O.85,88
Following intubation, hypotension primarily occurs secondary to hyperinflation, but also is exacerbated by a combination of sedation and hypovolemia.41
Controlled hypoventilation, also known as permissive hypercapnia, is essential in the immediate post-
intubation period to reduce hyperinflation. It often is combined with increased expiratory time and continuous bronchodilator therapy to decrease expiratory flow resistance.89,90
As previously mentioned, people with asthma are prone to hypotension for various reasons. In addition to treatment of the underlying asthma, the patient also must be assessed for volume status on arrival. Signs of poor hydration include severe respiratory distress, poor oral intake, and clinical signs of dehydration, such as dry mucous membranes, poor skin turgor, and minimal urine output. Dehydration can lead to hypotension, while fluid overload can lead to pulmonary edema.67 Once the patient has improved clinically to the point of comfort with at least mild activity, it is reasonable to consider an oral fluid challenge.
Children with severe acute asthma often are dehydrated from poor oral intake and increased insensible fluid losses. Fluid resuscitation often is indicated. This aid depends on the disposition of the patient, particularly young asthmatics. If the patient is not tolerating oral fluids and has signs of dehydration, it is reasonable to give a 20 mL/kg normal saline bolus, followed by maintenance fluids with likely admission.
Discharge
Comprehensive discharge planning is essential for pediatric patients treated for acute asthma in the ED. The CHOP pathway emphasizes family viewing of an asthma education video, consideration of starting a controller medication, MDI instruction, follow-up appointments with a primary care physician, and return precautions. MDIs with holding chambers produce outcomes that are at least equivalent to nebulizer therapy for inhaled corticosteroids and beta agonists in acute asthma. Proper MDI use might prevent ED visits for some mild-to-moderate cases of acute asthma, which further highlights the importance of proper MDI use.91 Zorc et al found that scheduling an appointment after an ED visit increased the likelihood that urban children with asthma would follow up with a provider.92
Follow-up with the patient’s primary care physician is recommended within three to five days and provides an opportunity to discuss an asthma plan and possible controller medication therapy, which may reduce future acute exacerbations.86
The outpatient follow-up rates after ED visits for acute asthma remain low.93 One solution is to prescribe or provide an inhaled corticosteroid controller medication in the ED at discharge. It has been suggested that this approach is more cost-effective than relying on outpatient follow-up, reduces bounce-back visits to the ED, and results in higher rates of controller medication initiation.93,94
The authors of a recent study found there was an increased likelihood of follow-up with primary care physicians within four weeks of the ED visit if parents believed their child had “very severe” asthma and was using a daily controller medication.95
Furthermore, more than 80% of pediatricians support initiation of corticosteroid controller medications in the ED.96 Another proactive measure that can be implemented in the ED is influenza vaccination, since vaccination rates among children with asthma remains low despite increased complication rates among those with asthma.97,98 Children with asthma represent more than one-third of pediatric patients admitted for influenza, despite an asthma prevalence rate of 10%.98
Conclusion
Acute asthma exacerbations represent a large proportion of ED visits among children — with significant mortality and cost to the healthcare system. The implementation of standardized pathways incorporating validated asthma severity scoring systems continues to expand, with an emphasis on rapid triage and early goal-directed therapy involving steroids and bronchodilator therapy. The role of chest X-rays continues to diminish while point-of-care ultrasound use increases. Patients with severe symptoms should receive appropriate oxygen and pharmacologic therapy, including the use of ketamine prior to noninvasive or invasive ventilation.
However, progression to intubation should not be delayed in patients with worsening hypercarbic and hypoxic respiratory failure and with altered mental status. When discharging patients, provide appropriate follow-up and instruction, and prescribe controller medications for patients with persistent features.
References
A complete list of references can be found online at https://bit.ly/2MLkj3d.
Asthma is the most common chronic disease of childhood. Children with asthma frequently present in the acute care setting with disease ranging from mild to severe. Accurately assessing children with asthma and providing escalating care as needed improves outcome. The authors provide a current review of asthma and evidence-based care.
Subscribe Now for Access
You have reached your article limit for the month. We hope you found our articles both enjoyable and insightful. For information on new subscriptions, product trials, alternative billing arrangements or group and site discounts please call 800-688-2421. We look forward to having you as a long-term member of the Relias Media community.
