Trauma in Pregnancy: A Comprehensive Overview
May 1, 2020
Related Articles
-
Echocardiographic Estimation of Left Atrial Pressure in Atrial Fibrillation Patients
-
Philadelphia Jury Awards $6.8M After Hospital Fails to Find Stomach Perforation
-
Pennsylvania Court Affirms $8 Million Verdict for Failure To Repair Uterine Artery
-
Older Physicians May Need Attention to Ensure Patient Safety
-
Documentation Huddles Improve Quality and Safety
AUTHORS
R. Gentry Wilkerson, MD, Assistant Professor, University of Maryland School of Medicine, Department
of Emergency Medicine, Baltimore
Sharleen Yuan, MD, MA, PhD, University of Maryland School of Medicine, Department of Emergency Medicine, Baltimore
T. Andrew Windsor, MD, Assistant Professor, University of Maryland School of Medicine, Department of Emergency Medicine, Baltimore
PEER REVIEWER
Dennis Hanlon, MD, FAAEM, Allegheny General Hospital, Medical Director, Department of Emergency Medicine
EXECUTIVE SUMMARY
• During the late third trimester, the diaphragm can be displaced cephalad by as much as 4 cm, necessitating placement of a tube thoracostomy at a site one to two rib interspaces more superior than in a nonpregnant patient.
• Hemorrhagic shock may not develop until as much as 35% of blood volume is lost as a result of the physiologic changes of pregnancy.
• Pregnancy is considered a prothrombotic state with a four- to six-fold increased risk of venous thromboembolism during pregnancy and during the postpartum period.
• Any patient in the later stages of pregnancy should be transported in the left lateral decubitus position to prevent development of supine hypotension syndrome.
• Pregnant patients are less tolerant of prolonged apnea times because of reduced functional reserve capacity and increased oxygen demand; therefore, preoxygenation ideally should be performed if time allows, and continue the administration of oxygen using a nasal cannula during intubation to increase the time to desaturation.
• A viable fetus (usually > 22 to 24 weeks) should receive continuous external fetal monitoring throughout all procedures and management as long as such monitoring does not interfere with treating the mother.
• The standard dose of 300 mcg of anti-D IgG is effective at preventing sensitization to as much as 30 mL of fetal whole blood. If the Kleihauer-Betke test indicates that more than 30 mL of fetal whole blood is present in maternal circulation, then an appropriate amount of additional anti-D IgG should be administered.
The authors provide a concise, comprehensive overview of the unique anatomic and physiologic features of pregnancy, as well as modifications and considerations important for the management of the pregnant trauma patient.
— Ann M. Dietrich, MD, Editor
Introduction
The approach to the care of a pregnant patient who has sustained traumatic injuries requires special consideration and understanding of the unique challenges presented by this vulnerable population who have altered anatomy and physiology. The provider also must be aware that, in addition to the usual traumatic injuries experienced by all trauma patients, pregnant patients may sustain injuries unique to pregnancy, such as placental abruption, uterine rupture, and preterm labor. Additionally, delivery after a traumatic event carries significant risk of morbidity and mortality for both the mother and fetus. A major challenge for the provider is that a pregnant patient presents two patients for the clinician to care for simultaneously, although the well-being of the fetus is generally predicated on and secondary to aggressive and successful resuscitation of the mother. Many emergency departments or prehospital jurisdictions follow the good practice of having specific algorithms for the management of trauma in pregnancy. Providers should familiarize themselves with their own institutional or regional variation in practice. This article will provide an overview of general management and a description of the pathophysiology encountered with the pregnant trauma patient.
Epidemiology
Approximately one in 12 pregnancies is complicated by a traumatic injury, with trauma being the most frequent nonobstetric cause of maternal mortality.1,2 The actual incidence of trauma during pregnancy is likely much higher because of underreporting of nonfatal trauma. Injuries are the proximate cause of 20% of maternal deaths.3 Mortality due to trauma is not included in the commonly used maternal mortality ratio and pregnancy-related mortality ratio statistics, leading to limited recognition of the problem’s magnitude.4 In analyzing data from the Pennsylvania Trauma Outcome Study, Deshpande et al found that, overall, the most common cause of trauma in pregnant women is motor vehicle collisions (58.1%), followed by falls (16.7%) and assaults (14.9%). Similar to nonpregnant women of reproductive age, blunt trauma accounted for 88% of injuries while penetrating trauma and burns caused 7% and 4%, respectively. Pregnant patients are more likely to experience violent trauma as compared to nonpregnant patients (15.9% vs. 9.8%). In the study by Deshpande et al, pregnancy also was associated with a 1.6-fold higher rate of mortality for traumatic events.5 A recent systematic review found that intimate partner violence and domestic violence, including verbal abuse, is reported to occur in up to 57% of all pregnancies.6 In an Australian population, exposure to intimate partner violence with injury led to a 1.7-fold increase in the rate of maternal complications and a two-fold higher rate of adverse fetal outcomes.7 Risk factors for experiencing violent trauma include younger age, African American race, being employed, public or no insurance, history of psychiatric or seizure disorder, and history of drug or alcohol abuse.5,8
Pregnancy may be an incidental finding during the evaluation of a patient with traumatic injuries. A single-center, retrospective study of female patients ages 15 to 40 years who received care at R Adams Cowley Shock Trauma Center at the University of Maryland Medical Center found that the prevalence of pregnancy was 3%. Further, in 8% of these pregnant trauma patients, the pregnancy was diagnosed during the trauma evaluation.9
Anatomic and Physiologic Changes of Pregnancy
Pregnancy results in alterations in physiology and differences in anatomic positioning of structures within the abdomen and pelvis. These changes lead to differences in the signs and symptoms that develop as a result of traumatic injury as well as differences in the pattern of injury. (See Table 1.)
Table 1. Key Physiologic Changes in Pregnancy |
|
Cardiovascular |
|
|
Respiratory |
|
|
Hematologic |
|
|
Gastrointestinal |
|
|
Renal |
|
Anatomic Changes
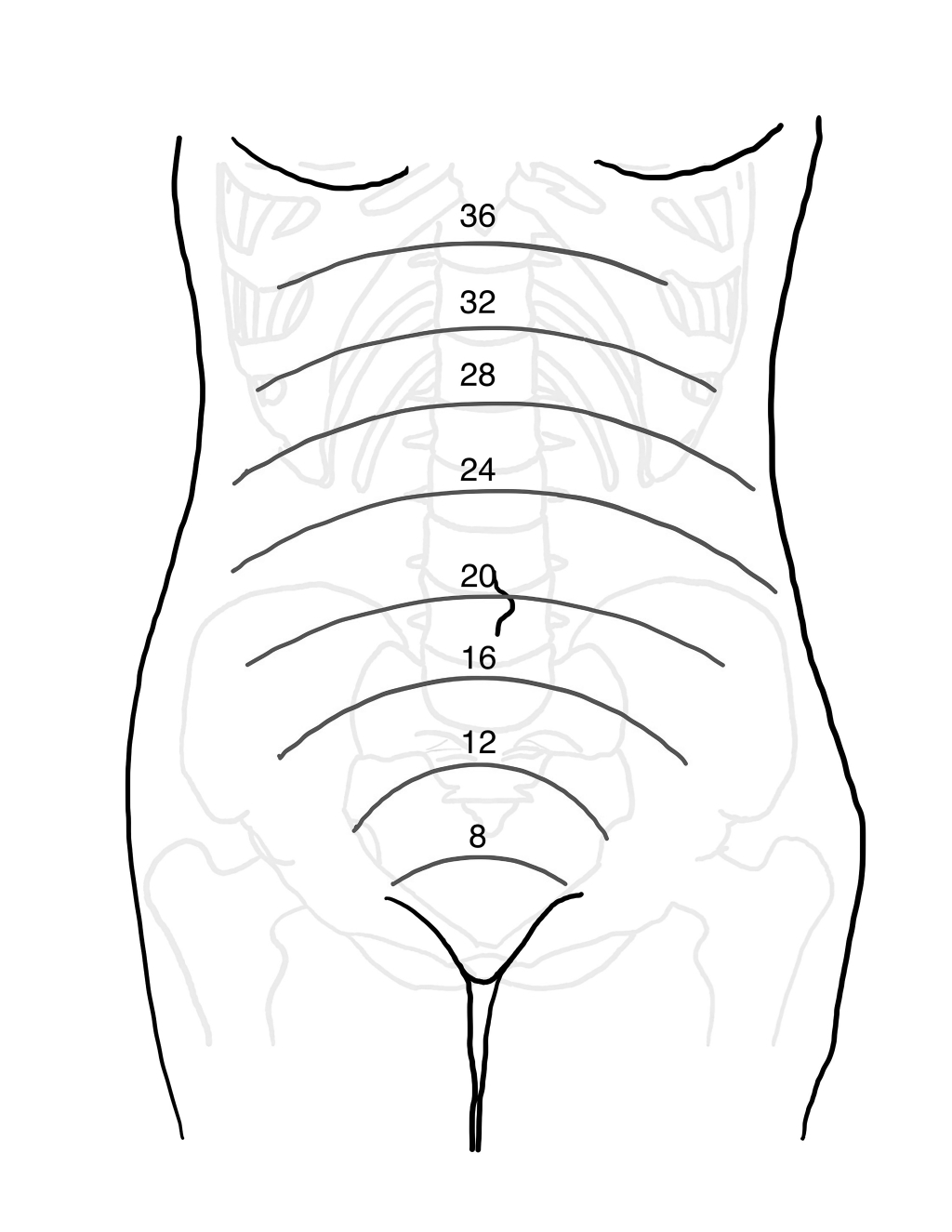
The uterus increases in size corresponding to the growth and development of the fetus. (See Figure 1.) During the first 12 weeks of pregnancy, the uterus remains within the pelvis. The uterus reaches the level of the umbilicus at approximately 20 weeks and then the costal margin between 34 and 38 weeks. As the uterus grows, there is cephalad displacement of intraabdominal organs. During the first trimester, the bony pelvis and the thick-walled uterus provide relative protection to the fetus. As the uterus increases in size, it can compress adjacent structures, such as the inferior vena cava. During the late third trimester, the diaphragm can be displaced cephalad by as much as 4 cm. This alteration necessitates placement of a tube thoracostomy at a site one to two rib interspaces more superior than in a nonpregnant patient.10 As a result of upward displacement into the relatively protected thoracic space, there is some protection of the bowel in cases of blunt abdominal trauma.
Figure 1. Approximate Fundal Height by Weeks of Gestation |
 |
Cardiovascular Changes
During pregnancy, cardiac output increases (up to 40%) because of an increase in heart rate by 15-20 beats per minute and an increase in stroke volume.11 The mean arterial pressure falls as a result of decreases in both systolic and diastolic blood pressures (systolic pressure decreases 2 mmHg to 4 mmHg, diastolic pressure decreases 5 mmHg to 15 mmHg). Most decreases in blood pressure occur in the sixth to eighth week of gestation.12 Blood pressure decreases in the first trimester, stabilizes in the second trimester, and returns to nonpregnant-level baselines in the third trimester. A physiologic anemia of pregnancy develops because there is an increase in plasma volume of 40% to 50% accompanied by a 20% to 30% increase in red cell mass.13 Signs of hemorrhagic shock may not develop until as much as 35% of blood volume is lost as a result of these physiologic changes.14,15
Elevated blood pressure in a pregnant patient at 20 weeks or more gestational age should warrant evaluation for preeclampsia. Supine hypotensive syndrome (also referred to as aortocaval hypotensive syndrome) can occur as a result of compression of the inferior vena cava and/or aorta by the enlarged uterus. There are no accepted criteria for making the diagnosis of supine hypotensive syndrome, but Humphries et al defined it as a decrease in systolic blood pressure of 15 mmHg to 30 mmHg or an increase in heart rate of 20 beats per minute with or without the presence of symptoms.16 Pregnant patients should be placed in the left lateral decubitus position to minimize the occurrence of supine hypotension. The hemodynamics may improve once the compression is removed. Additionally, it is essential to maintain sufficient tissue perfusion to allow adequate oxygen delivery because blood flow to the pregnant uterus can increase to 600 mL/minute (compared to 60 mL/minute for the nonpregnant patient).17
Hematologic Changes
Erythrocyte and leukocyte production increases during pregnancy, starting in the first six to eight weeks of gestation and peaking around 34 weeks. Total blood volume can increase by as much as 45%. Platelet levels tend to decrease slightly during the third trimester. Thrombocytopenia that develops during the first trimester or a platelet count < 80,000/microL during any trimester warrants evaluation for immune thrombocytopenia.13 Pregnancy is considered a prothrombotic state with a four- to six-fold increased risk of venous thromboembolism (VTE) during pregnancy and the postpartum period.18 The underlying mechanism for increased VTE risk is a rise in procoagulant factors — such as factor I (fibrinogen), factor VII, factor VIII, factor X, and von Willebrand factor — and a decrease in anticoagulant factors, such as protein S, as well as a decrease in resistance to activated protein C.19
Pulmonary Changes
In addition to the cardiovascular changes noted earlier, pregnancy induces changes to the pulmonary system to meet the increased metabolic demands of the mother and maximize oxygen delivery to the fetus. With displacement of abdominal contents cephalad, thoracic space would decrease if compensatory mechanisms were not available. One such mechanism is an increase in diaphragm excursion during inspiration of approximately 2 cm. There also is an increase in the anteroposterior and transverse diameters of the chest wall. Functional reserve capacity decreases approximately 20% to 30%, leading to increased inspiratory capacity. As a result of these changes, the total lung capacity remains relatively unchanged until the third trimester, when there is a small decrease of about 5%. A 30% to 50% increase in tidal volume with minimal increase in breaths per minute results in increased resting minute ventilation.20
Upper airway structures also are altered in pregnancy. The mucosa becomes edematous, hyperemic, and friable. The late third trimester of pregnancy is associated with an increase in the Mallampati score.21 However, the Mallampati score, as well as other tests to determine the likelihood of successful intubation, performed poorly in a study of 239 patients undergoing emergency cesarean delivery.22
Renal Changes
Alterations in the renin-angiotensin-aldosterone system and other hormonal changes lead to intravascular volume expansion. The glomerular filtration rate progressively increases during pregnancy to 40% to 50% above baseline. This leads to decreases in serum levels of creatinine, blood urea nitrogen, and uric acid.23 Hydronephrosis and hydroureter without intraluminal obstruction are common findings, occurring in more than 80% of pregnancies.24
Gastrointestinal Changes
The elevation in pregnancy hormones, such as progesterone, lead to delayed gastric emptying and reduced lower esophageal sphincter tone.25 Placental production of gastrin leads to increased gastric acidity.26 These factors increase the risk of aspiration and development of aspiration-related complications.
Prehospital Care
The prehospital care of the pregnant trauma patient largely mirrors the care provided to the nonpregnant patient: rapid assessment, performance of necessary stabilization treatments, and transport to the closest appropriate medical facility. During the assessment phase, the history obtained should include as much obstetric history as possible that would be pertinent to the patient. An estimation of gestational age based on the date of the onset of the last menstrual period will help the destination facility determine the need for specific resources. Regardless of the presence of other indicators of trauma severity, pregnancy > 20 weeks is a criterion for transport to a trauma center according to the Centers for Disease Control and Prevention update on the “Guidelines for Field Triage of Injured Patients.”27 Any patient in the later stages of pregnancy should be transported in the left lateral decubitus position to prevent development of supine hypotension syndrome.16 Additionally, the mother should be placed on supplemental oxygen, and appropriate intravenous (IV) access should be obtained.
In the case of cardiac arrest, the 2015 American Heart Association guidelines for the management of cardiac arrest in pregnancy recommend placing the patient in the supine position with manual displacement of the uterus rather than placing the patient in the left lateral decubitus position. This is because of improved quality of chest compressions, lack of full aortocaval decompression when placed in the left lateral decubitus position, and improved conditions for other procedures associated with resuscitation (e.g., intubation). Use of two hands is required to manually displace the uterus to the left, toward the patient’s head, at a position between 15 and 30 degrees. Elevating the legs also will improve venous return.28
Emergency Department Assessment and Management
The primary focus of initial management of the pregnant trauma patient in the emergency department should be on the welfare of the mother rather than that of the fetus. The best initial treatment of the fetus is to stabilize the mother because fetal outcome is directly related to aggressive and early stabilization of the mother. Therefore, fetal assessment is deferred until the secondary survey. If possible, especially in a pregnancy of viable gestational age, the patient should be evaluated by the trauma and obstetrical teams in tandem, with the neonatal intensive care unit alerted to be prepared for infant resuscitation.
Primary Survey
The primary survey follows the general principles of the American College of Surgeons’ Advanced Trauma Life Support (ATLS) course with stepwise evaluation of “ABCDE”: airway, breathing, circulation, disability, and exposure/environmental control.10 For the pregnant patient, “D” also can stand for “displacement” of the uterus, as a reminder to position the patient or manually displace the uterus, which can help with maternal hemodynamics.
Evaluate the airway first to determine patency. The presence of foreign bodies or facial fractures can lead to obstruction. If there is doubt about airway patency, then a definitive airway should be established. Pregnant patients are less tolerant of prolonged apnea times because of reduced functional reserve capacity and increased oxygen demand; therefore, preoxygenation ideally should be performed if time allows. Continuing the administration of oxygen using a nasal cannula during intubation may increase the time to desaturation during the period of apnea.29 Difficult airways are encountered in approximately 5% of pregnant patients,30,31 similar to rates seen in all patients.32 The rate of failed tracheal intubation is many times higher in pregnant patients as compared to general surgical patients (0.4% vs. 0.04%); however, the overall rate remains low.33 Application of cricoid pressure often is recommended, but its utility is controversial.34,35 After intubation, an orogastric tube should be placed to allow for suctioning of gastric contents to reduce the risk of aspiration.3
The second step in the evaluation of the patient involves assessment of breathing to ensure adequate ventilation. The physiologic changes of pregnancy lead to a reduction in the partial pressure of carbon dioxide (PaCO2) level to between 27 mmHg and 34 mmHg.21 When PaCO2 levels in the pregnant patient are within ranges considered normal in the nonpregnant state, the patient actually may have impending respiratory failure. The most recent edition of the ATLS manual recommends the fourth or fifth intercostal space anterior to the mid-axillary line instead of the second intercostal space mid-clavicular line as the preferred site for needle decompression.10 If pneumothorax is suspected or confirmed, placement of a thoracostomy tube should be performed one to two rib interspaces superior to the usual location because of the diaphragm elevation seen in pregnancy.3
The third step in the evaluation of a trauma patient is circulation. Evaluation of the patient’s circulatory status focuses on detecting signs and symptoms of shock and identifying active sites of bleeding. Shock in a trauma patient commonly is the result of hemorrhage, direct cardiac injury, or mechanisms that reduce cardiac output, such as tamponade or tension pneumothorax. The patient should have adequate IV access established with two large-bore IV cannulas. The location of IV access is preferably above the diaphragm. To prevent aortocaval compression by the uterus, it should be displaced manually, or the patient should be placed in the left lateral decubitus position. Hemorrhagic shock can be treated initially with judicious administration of IV crystalloids until blood products are available. To avoid alloimmunization in Rh (-) females, uncrossmatched type O Rh (-) blood can be administered until type-specific blood is available.36 If there is clinical evidence that shock is caused by tamponade or tension pneumothorax, appropriate procedures should be performed without delay to relieve the cause of the obstruction.
Disability assessment is a rapid neurologic assessment to determine the patient’s level of consciousness. The Glasgow Coma Scale is a well-established, structured tool with adequate reliability to determine the level of consciousness using motor response, verbal response, and eye opening.37 Pupils should be examined to assess their size and responsiveness to light. Finally, the patient should be assessed for lateralizing signs by comparing movement and strength in the right vs. left extremities.
Exposure and environmental control are the final components of the primary survey. The patient should be fully exposed (undressed) to facilitate a thorough assessment. The patient should be kept covered with warm blankets when not being examined to prevent hypothermia. Hypothermia is a component of the “trauma triad of death” that also includes coagulopathy and acidosis. The three components of the triad are linked and result in a vicious cycle where each one serves to worsen the others.38
Secondary Survey
The secondary survey, during which a head-to-toe evaluation of the patient occurs, does not begin until after the primary survey has been completed. During the secondary survey, a complete history is obtained, vital signs are reassessed, and a thorough physical examination is performed. Frequently, the mnemonic AMPLE (allergies, medications, past illnesses/pregnancy, last meal, events/environment related to the injury) is used to help guide the history-taking process.39 The obstetric history should include past pregnancies, current gestational age, estimated date of delivery, prenatal care received, and any known complications, such as preeclampsia or placenta previa. Initial evaluation of the fetus does not occur until during the secondary survey. At this time, the fetus can be assessed using ultrasound to determine fetal heart rate and movement. A viable fetus (usually > 22 to 24 weeks) should receive continuous external fetal monitoring throughout all procedures and management as long as such monitoring does not interfere with treating the mother. Once the mother is stabilized, cardiotocographic monitoring should be performed for at least four hours with extension to 24 hours if necessary, based on the presence of high-risk features, such as major maternal trauma, uterine contractions or tenderness, vaginal bleeding, rupture of membranes, or the presence of nonreassuring fetal heart tones.40
Physical examination of the pregnant trauma patient is similar to that of the nonpregnant patient. Because of the anatomic changes that occur in pregnancy, the abdominal examination can be challenging and, often, unreliable. Uterine tenderness increases the suspicion for placental abruption or uterine rupture. In cases where the gestational age is > 23 weeks, an internal pelvic examination should be deferred until placenta previa can be ruled out by ultrasound. A sterile speculum examination is indicated if there is evidence of vaginal bleeding, fluid leakage, uterine tenderness, or contractions. To reduce the risk of infection, bimanual examination should be deferred if there is concern that membranes have ruptured. Pooling of amniotic fluid in the posterior vaginal vault is indicative of rupture of the chorioamniotic membrane. The vagina also should be examined for the presence of bony fragments or vaginal lacerations. A bedside ultrasound can be used to assess for gestational age, placental location, fetal presentation, cardiac activity, and fluid volume. The probe itself is an excellent tool to assess for uterine tenderness. If biometric calculation is not available, fetus femur length can be used, with length > 4 cm indicating fetal viability.39
Laboratory Testing
Laboratory testing in the pregnant trauma patient is similar to that of the nonpregnant patient. Most laboratory testing in the trauma patient is done by protocol. The clinician should be aware of the usual physiologic changes that occur in pregnancy that may alter the normal results of various tests.
Type and Screen
When the pregnant trauma patient arrives, a type and screen should be sent to help guide blood product type in case blood products need to be administered. Additionally, testing is performed to determine the patient’s Rh status, which is important because all Rh (-) mothers with abdominal trauma are at risk for alloimmunization resulting from fetomaternal hemorrhage (FMH). Rh (-) patients at risk for FMH should be treated with a standard dose of 300 mcg Rho(D) immune globulin G (anti-D IgG, RhoGAM) within 72 hours to prevent alloimmunization. This dose will provide protection for up to 30 mL of fetal whole blood in the maternal circulation. Blunt abdominal trauma in a pregnant patient increases the likelihood of higher-volume FMH, and thus the need for higher doses of anti-D IgG.41
Coagulation Profile and Fibrinogen Levels
Laboratory testing of the coagulation profile with fibrinogen levels is useful in evaluating a patient at risk of developing disseminated intravascular coagulation. Normally, during pregnancy, the levels of fibrinogen are elevated and may be as high as 6 g/L.42,43 Fibrinogen levels that are normal or low may be an early indicator of the development of disseminated intravascular coagulation. Fibrinogen levels < 2 g/L have been reported to have a 100% positive predictive value for the diagnosis of severe postpartum hemorrhage.44 This suggests reasonable utility of fibrinogen testing to detect severe traumatic hemorrhage.
Kleihauer-Betke Test
The Kleihauer-Betke (KB) test is used to detect the presence of fetal blood cells in maternal circulation based on differences in susceptibilities of the red blood cells of the mother and the fetus to acid elution. Cells with higher levels of fetal hemoglobin (HbF) are resistant to acid elution, whereas cells with little or no HbF are sensitive. To perform the test, a thin smear of the maternal blood is prepared and then exposed to an acid buffer. The slide then is stained with hematoxylin so that fetal cells are dark pink and maternal cells are pale and “ghost”-like. A formula frequently used to calculate the volume of fetal whole blood is:
% of fetal cells determined by KB test /100 × 5,000 mL = volume of FMH (in mL).45
The KB test is used to help guide the need for additional doses of anti-D IgG. It does not rule out the presence of FHM since the detection limits are above the amount that is required for alloimmunization. The standard dose of 300 mcg of anti-D IgG is effective at preventing sensitization to as much as 30 mL of fetal whole blood. If the KB test indicates that more than 30 mL of fetal whole blood is present in maternal circulation, then an appropriate amount of additional anti-D IgG should be administered.
Although the KB test is used widely to determine the dose of anti-D IgG, the test has relatively poor accuracy and reproducibility.46 The test is complicated to perform and labor-intensive, and multiple factors may result in under- or overestimation of the volume of fetal blood. Underestimation may occur as a result of decreasing amounts of HbF in fetal blood as gestational age increases. At term, HbF may account for as little as 70% of the hemoglobin present in fetal blood. Underestimation also may occur in cases where maternal alloimmunization has previously occurred and there is removal of fetal blood cells by the maternal reticuloendothelial system. Overestimation may occur in cases where high levels of HbF are present in maternal red blood cells, such as in patients with sickle cell disease and especially if these patients are treated with hydroxyurea. Overestimation also may occur as a result of naturally increasing levels of HbF as gestational age increases.47
Some have advocated for using KB testing in all pregnant trauma patients regardless of Rh status.48-50 Their assumption is that the KB test will serve as a good screening tool to identify significant trauma to the uterus that would result in fetal distress or development of placental abruption. However, there is no evidence to suggest that KB testing is more accurate than fetal monitoring to detect fetal distress.3 A recent study assessed the results of KB testing in 68 cases of proven abruption. The KB test was positive in only three cases of abruption, giving a sensitivity of only 4.4%.51 Despite this, a recent survey of laboratories primarily located in the United States revealed that 52.2% were performing FMH testing on Rh (+) females.47
Flow Cytometry
Flow cytometry is a novel alternative to the KB test to quantify fetal red blood cells present in maternal circulation. Flow cytometry has superior test characteristics when compared to KB testing. It is more sensitive and objective, and it provides quicker results.52,53 The major constraint on the use of flow cytometry at this time is the limited availability of this test. A 2019 survey found that cytometry testing was performed in only 4.4% of laboratories.47
Testing for Prelabor Rupture of Membranes
Prelabor rupture of membranes (also known as premature rupture of membranes),54 the rupture of the chorioamniotic membrane prior to the onset of labor, may be evidenced by fluid pooling in the vagina or fluid leakage from the cervical os. Fluid present in the posterior fornix of the vagina can be collected and tested for pH and the presence of ferning. In the fern test, secretions are placed onto a glass microscope slide, allowed to dry, and then viewed on a microscope to look for a characteristic arborization pattern. Normal vaginal secretions typically have a slightly acidic pH of 5. Vaginal fluid with a pH of 7 is suggestive of an amniotic source. Several commercially available immunoassay tests can detect the presence of amniotic fluid proteins to assist in the recognition of amniotic fluid. These tests have sensitivities and specificities that are above 90%. However, in the presence of blood, the ROM Plus test performed significantly better than the Actim PROM and AmniSure tests.55
Imaging Studies
The evaluation of the pregnant trauma patient often will use a combination of ultrasound, plain film radiography, and computed tomography (CT) to evaluate for injuries. Magnetic resonance imaging (MRI) has a limited role in the initial evaluation of any trauma patient because of the length of time required to complete the examination and the lack of proximity of most MRI machines to the clinicians caring for the patient.
The clinician must balance the need for imaging with the judicious use of ionizing radiation through the practice of the as low as reasonably achievable (ALARA) principle.56 The risk of radiation to the fetus depends on the dose of radiation and the stage of the pregnancy. (See Table 2.) Risks include malformation, pregnancy loss, increased lifetime risk of cancer, and decreased intelligence. The risk of radiation-induced malformations is greatest during the period of organogenesis and early fetal development from weeks 2 through 15.57 The National Council on Radiation Protection and Measurements published a report in 1977 that included the statement that the risk of radiation causing fetal abnormality was negligible at doses less than 50 mGy (5 rad).58 In 2004, the American College of Obstetricians and Gynecologists supported the use of the 5 rad cutoff.59 Stochastic carcinogenic effects of radiation, by definition, are random and have no threshold value. According to the American College of Radiology, there is a 0.4% increase in the cumulative lifetime incidence of cancer when a fetus is exposed to 10 mGy (1 rad).60
Table 2. Estimated Fetal Radiation Dose From Select Radiographic Studies |
||
|
Imaging Study |
Radiation Dose (rad) |
Number of Studies Needed to Reach 5-rad |
|
Chest radiograph (two views) |
0.00005 to 0.001 |
5,000 to 100,000 |
|
Upper or lower extremity radiograph |
< 0.0001 |
> 50,000 |
|
Cervical spine radiograph |
< 0.0001 |
> 50,000 |
|
Abdominal radiograph |
0.01 to 0.3 |
17 to 500 |
|
Intravenous pyelography |
0.5 to 1 |
5 to 10 |
|
CT, head (10 slices) |
0.0001 to 0.001 |
5,000 to 50,000 |
|
CT, chest (10 slices) |
0.001 to 0.066 |
75 to 5,000 |
|
CT, abdomen (10 slices) |
0.13 to 3.5 |
2 to 38 |
|
CT = computed tomography Adapted from: Committee on Obstetric Practice. Committee Opinion No. 723: Guidelines for diagnostic imaging during pregnancy and lactation. Obstet Gynecol 2017;130:e210-e216. |
||
Frequently, lead shielding will be used in an effort to reduce the fetus’s exposure to radiation. The utility of this practice has been questioned.59,61 Technological advances have substantially reduced external scatter of radiation outside of the field of view. For imaging studies that do not involve the uterus within the field of view, most of the scatter to which the fetus is exposed is the result of internal scatter. External shielding has no effect on internal scatter.60 Using a phantom simulation, one group calculated that using lead shielding of the abdomen during posteroanterior chest radiograph acquisition would reduce the total fetal radiation exposure by only 4%.62 In another phantom simulation study of pediatric chest CT, the use of lead shielding was associated with a < 1% reduction in radiation exposure.63
The indications for imaging in the pregnant trauma patient generally are the same as for the nonpregnant patient. Multiple guidelines and recommendations support the appropriate use of imaging studies in the evaluation of a pregnant trauma patient.3,64 However, the concern for exposure of the fetus to ionizing radiation may lead the clinician to stray from accepted practice in the evaluation of the pregnant trauma patient. In a single-center study performed in Australia that included 32 patients with high-risk trauma, the compliance with imaging guidelines was only 18.8%.65
Ultrasound
Frequently, the first examination performed on a pregnant trauma patient is the focused assessment with sonography for trauma (FAST), without or with the inclusion of lung windows (the latter is the extended FAST or eFAST examination). The FAST examination was designed to detect free fluid in the abdomen suggestive of intraabdominal injury, pericardial effusion, and pleural effusion. Performing the FAST examination requires obtaining views in four locations: the subxiphoid, the right and left upper quadrant, and the suprapubic region. A positive FAST examination in an unstable patient is an indication for immediate exploratory laparotomy.
It is important to remember that a negative FAST examination does not rule out injury. A recent meta-analysis reported the summary sensitivity of 34 included studies to be 0.74 (95% confidence interval [CI], 0.65-0.81).66 FAST is less accurate in assessing pregnant trauma patients than nonpregnant ones.67 Performing serial ultrasound assessments if the first examination is negative has been shown to increase the sensitivity.68 Pregnant patients are at increased risk of retroperitoneal hemorrhage due to rupture of the pelvic plexus and an increase in the transmission of shearing forces through the uterus.50,69 Ultrasound is not useful for the assessment of retroperitoneal hemorrhage70 and is not sensitive enough to exclude placental abruption.66
As mentioned previously, the eFAST examination includes scanning of the lungs. A bedside ultrasound of the lungs can be performed by an emergency physician and has higher sensitivity and similar specificity for the detection of pneumothorax as compared to supine anteroposterior chest radiographs (sensitivity 86% to 98% vs. 28% to 75%, and specificity 97% to 100% vs. 100%).71 Other uses of ultrasound in the assessment of pregnant trauma patients include assessment of fetal heart rate, movement, gestational age, and presentation, as well as the quantity of amniotic fluid and placental position. In the third trimester, the most accurate measurement for estimating gestational age is the fetal femur length.72
Plain Film Radiographs
Plain film radiographs are obtained frequently to evaluate trauma to the extremities. During the initial evaluation of a patient with a traumatic injury, algorithms often call for plain film radiographs of the cervical spine, chest, and pelvis. In the case of a pregnant patient, the provider may consider deferring these studies if they will be redundant to additional imaging obtained with CT.
CT Imaging
CT imaging uses a much greater amount of ionizing radiation than plain film radiography during image acquisition, thus increasing fetal exposure. Using iodinated contrast media during CT imaging aids in the diagnosis of various conditions. Prior to 2015, when the lettering system was replaced, the U.S. Food and Drug Administration classified most iodinated contrast media as pregnancy category B.73 Medications were given pregnancy category B if “animal reproduction studies have failed to demonstrate a risk to the fetus, and there are no adequate and well-controlled studies in pregnant women.”74 Contrast-enhanced CT imaging can aid in the diagnosis of placental abruption, one of the major complications of trauma in pregnant patients. Placental abruption has been associated with CT imaging, demonstrating a 50% decrease in placental perfusion.75,76
Fetal Monitoring
After the primary survey is completed, if the mother is stable, cardiotocographic monitoring should be performed. External fetal monitoring with cardiotocography allows for continuous visualization of fetal heart activity and of uterine contraction, both of which can be indicative of potential obstetrical complications as a result of traumatic injury. Monitoring generally is recommended for viable pregnancies and serves little purpose, even in cases of fetal distress, for nonviable pregnancies that will not be delivered. Abnormal readings are insensitive for predicting adverse fetal outcomes, but should warrant further evaluation and treatment, closer monitoring, or possibly even delivery. Fetal monitoring should occur for a minimum of four hours. The duration of monitoring should be extended to 24 hours if there is evidence of high-risk features, such as major maternal trauma, uterine contractions or tenderness, vaginal bleeding, rupture of membranes, or the presence of nonreassuring fetal heart tones.40
Injuries that lead to alteration of fetal perfusion or oxygen delivery often present with changes in the fetal heart rate pattern. The normal fetal heart rate ranges from 120 to 160 beats per minute. Hemodynamic instability of the fetus can be seen with late decelerations, tachycardia, or bradycardia. Additionally, a loss of variability in the heart rate is a sign of fetal central nervous system depression, indicating fetal distress.
Infrequent uterine contractions usually will self-resolve without adverse outcomes.50 However, frequent or intense contractions may be indicative of preterm labor or traumatic complications like placental abruption.
Medications
Rho(D) Immune Globulin G
Rho(D) immune globulin G is used to prevent alloimmunization in an Rh (-) mother who may have been exposed to blood from an Rh (+) fetus. If an Rh (-) mother is exposed to Rh (+) blood, the mother can create antibodies that could endanger future pregnancies. Anti-D IgG is a part of normal preventive care for Rh (-) women with healthy pregnancies. It also is recommended in other nontraumatic complications of pregnancy in Rh (-) women, such as threatened or actual miscarriage or performance of the external cephalic version procedure, where the provider attempts to rotate a fetus with breech presentation through external manipulation of the uterus.36
Because as little as 0.01 mL of Rh (+) blood can cause sensitization in up to 70% of Rh (-) patients, it is recommended that all Rh (-) trauma patients receive anti-D IgG unless the injury is isolated and distant from the uterus.10 In the setting of trauma, anti-D IgG should be administered within 72 hours of an injury that may have resulted in FMH. The standard initial dose of 300 mcg is effective at preventing sensitization to as much as 30 mL of fetal whole blood. If the testing indicates that more than 30 mL of fetal whole blood is present in maternal circulation, then an appropriate amount of additional anti-D IgG should be administered. A KB test or flow cytometry can help guide the need for additional doses.
Anti-D IgG is given via intramuscular injection. It is supplied as a prefilled syringe containing 300 mcg of anti-D IgG. A product containing 50 mcg also is available (MICRhoGAM). In cases of larger-volume FMH requiring multiple doses of anti-D IgG, the doses can be spaced out over time as long as the entire recommended dosage is given within 72 hours.77
Corticosteroids
In the event of expected or imminent preterm delivery for a viable fetus between 24 and 34 weeks gestational age, corticosteroids should be administered to the mother to aid in fetal maturation before birth. Corticosteroid administration is one of the more important therapies in preterm delivery and has been shown to reduce overall neonatal mortality, respiratory distress syndrome, intraventricular hemorrhage, and development of necrotizing enterocolitis.78 Typically, betamethasone or dexamethasone is administered in consultation with obstetrics and neonatology, before birth, with even a single dose of corticosteroid positively affecting fetal morbidity and mortality.79 Betamethasone also may confer a benefit if given to late preterm pregnancies between 34 and 37 weeks.80
Tocolytics
The use of tocolytics to delay delivery in the preterm pregnant trauma patient is controversial and, if being considered, is managed best by the consulting obstetrician. Sometimes, proceeding with delivery is in the best interest of the mother and possibly the fetus. If the membranes have not ruptured and placental abruption is considered to be unlikely, it is possible that tocolytics may aid in delaying delivery. This can allow administration of medications that may improve outcomes, such as magnesium sulfate for fetal neuro-protection if the fetus is less than 32 weeks gestational age, or corticosteroids for fetal maturation.81 The use of betamimetics, such as terbutaline, should be avoided as tocolytics in the pregnant trauma patient. This is because the drugs’ secondary hemodynamic effects, such as tachycardia or hypotension, may hinder resuscitation of the mother.40
Tranexamic Acid
Tranexamic acid (TXA) is a synthetic form of the amino acid lysine that was first synthesized in 1962 by Japanese researchers investigating potential antifibrinolytic treatments. The primary mechanism of action is the competitive binding of lysine receptor sites on both plasminogen and plasmin. When TXA binds to lysine receptor sites, it inhibits the conversion of plasminogen to plasmin and prevents converted plasmin from binding and degrading fibrin, thus preserving any clot matrix already formed.
Following publication of the results of the CRASH-2 clinical trial, TXA has been used increasingly in cases of traumatic injury. In this large clinical trial, patients sustaining trauma were randomized to receive either TXA as a loading dose of 1 g infused over 10 minutes, followed by an infusion of 1 g over eight hours, or matching placebo (0.9% saline) within eight hours of injury. Administration of TXA was shown to reduce all-cause mortality by 1.5% (14.5% in the TXA group vs. 16.0% in the placebo group) and death due to bleeding (4.9% vs. 5.7%).82 However, a subsequent analysis of the CRASH-2 data revealed an increase in death due to bleeding if TXA was administered more than three hours from the time of injury.83 Further evidence for the use of TXA in trauma came with the publication of the MATTERs study, a retrospective comparison of patients who received TXA compared to those who did not in the setting of trauma in a military hospital in Afghanistan. In this study, the use of TXA was associated with a reduction in unadjusted mortality (TXA 17.4% vs. no TXA 23.9%).84 Neither the CRASH-2 nor the MATTERs study specifically excluded pregnant women. There is no mention in either study of whether pregnant women were included.
The World Maternal Antifibrinolytic (WOMAN) trial, published in 2017, found that the use of TXA was associated with a reduction in the revised primary outcome of death due to postpartum hemorrhage (TXA 1.5% vs. placebo 1.9%). Most of the derived benefit was seen in patients who received the drug between one and three hours after delivery.85
The risk of VTE is not fully known. No increased risk of VTE was noted in the CRASH-2, MATTERs, or WOMAN trials. A meta-analysis published in 2017 that included eight studies (two prospective, six observational) found a nonsignificant increased risk ratio for the development of VTE with the administration of TXA (relative risk 1.32; 95% CI, 0.80-2.16).86 Following the publication of that meta-analysis, a single-center, retrospective analysis found a three-fold increase in the adjusted odds ratio for the development of VTE after administration of TXA (adjusted odds ratio 3.3; 95% CI, 1.3-9.1).87
Complications Specific to Trauma in Pregnancy
Placental Abruption
Placental abruption is a premature separation of the placenta from the uterus. Placental abruptions are seen most often in the setting of trauma, especially in abdominal trauma. As pregnancy progresses, the more muscular and pliable uterus absorbs much of the energy imparted from movement or impact, but some is transmitted to the placenta, which is more rigid. This tissue relationship gradient can lead to a shearing separation with a force that is significant enough. The severity of the trauma is correlated with the increased risk of placental abruption,88 which is the second leading cause of fetal death in trauma.39 Ultrasound’s importance in trauma is without question. Unfortunately, its utility in ruling out placental abruption is limited, with a sensitivity of about 25%, because 40% of abruptions involve concealed retroplacental hemorrhages.89 CT imaging has a sensitivity of 96% in diagnosing placental abruptions.90 However, most diagnoses are made using routine posttraumatic fetal cardiotocographic monitoring.84,86 Prompt awareness of placental abruption is key, as perinatal mortality can be as high as 53%, with near-miss maternal morbidity around 22%.87
Uterine Rupture
Uterine rupture is a rare complication, occurring in less than 1% of cases of trauma in pregnancy, but it has dire consequences for the fetus. Although maternal mortality as a result of uterine rupture is only about 10%, fetal mortality approaches 100%.40 Uterine rupture should be suspected if the uterus shape is irregular with palpation, fetal parts can be clearly palpated, there is severe abdominal tenderness, or extended fetal extremities are seen on radiography. The treatment for uterine rupture is surgical, with exploratory laparotomy used to deliver the fetus and to perform either hysterectomy or uterine repair. The primary risk factor for uterine rupture is prior cesarean delivery.68
Amniotic Fluid Embolism
Amniotic fluid embolism (AFE) is a rare life-threatening complication. Although not fully understood, AFE is thought to be due to amniotic fluid entering the maternal circulation through disruption of the maternal/fetal placental barrier, which can occur in trauma. There are two main hypotheses for the resultant effects: physical obstruction similar to other embolic disease, or a hypersensitivity/anaphylactoid-type reaction. AFE should be suspected if the patient has sudden cardiovascular collapse or hypoxemia without another explained cause, especially in the setting of multiorgan failure or disseminated intravascular coagulation. There is no definitive confirmatory test. The treatment of AFE is supportive and resuscitative, including possible delivery.91
Preterm Labor, Preterm Premature Rupture of Membranes, and Prelabor Rupture of Membranes
Trauma during pregnancy increases the risk of complications such as preterm labor, preterm premature rupture of membranes, and prelabor rupture of membranes, even beyond the initial date of injury. In one retrospective analysis of pregnant patients with a traumatic injury who were discharged home with a viable fetus, there was a two-fold increased risk of preterm delivery and low birth weight when compared to uninjured pregnancies.92 Suspicion for prelabor rupture of membranes should be heightened when there is pooling of fluid in the vagina or fluid leakage from the cervical os. This fluid should be collected and tested for ferning and an elevated pH, as described in the Testing for Prelabor Rupture of Membranes section. Historically, many guidelines discouraged using tocolytics to delay delivery if preterm labor develops in the setting of trauma. Now, if there is low clinical suspicion for placental abruption and the membranes are intact, short-term administration of tocolytics is considered acceptable, especially to enable administration of corticosteroids for lung maturation.40
Perimortem Cesarean Delivery
In the event of maternal cardiac arrest, perimortem cesarean delivery potentially can be lifesaving for both the mother and the fetus. Delivery of the fetus can improve hemodynamics to the mother by relieving compression of the inferior vena cava by a gravid uterus, as well as by eliminating the shunting of blood from the maternal circulation to the placenta and fetus. Perimortem cesarean delivery also has been called a resuscitative hysterotomy. Conventional cardiopulmonary resuscitation also is minimally effective in providing adequate perfusion to the fetus, so rapid delivery of a viable fetus gives the highest chance of survival of the child in the event of maternal demise. In general, it is recommended that perimortem cesarean delivery be considered in women at least 24 weeks pregnant.64 If gestational age is not known, palpation of the uterine fundus above the umbilicus or an ultrasound measurement of the fetal femur length > 4 cm is an adequate surrogate marker of viability.
Perimortem cesarean delivery is in the realm of “heroic” procedures, and most emergency providers will perform it rarely, if at all. One of the most important — and possibly challenging — steps is making the decision to go forward with the procedure. The earlier the delivery, the higher the likelihood that both the mother and fetus will survive, so the decision must be made quickly. The traditional teaching is that the procedure should commence within four minutes of maternal arrest, with delivery within the next minute. However, evidence suggests that this time course likely is unachievable even for trained obstetricians in an operating room setting.93,94 The key point is that sooner is better, with the completion of fetal delivery in less than five minutes likely resulting in the best outcome for both mother and fetus. Fetal survival has been reported even out to 30 minutes.95 However, as the mother hemorrhages, the fetus likely already has experienced a period of prolonged and worsening hypoxia, and limited data exist for outcomes related to hypovolemic cardiac arrest (as is most likely the case in the setting of trauma).
If maternal decompensation is expected or imminent, mobilization of resources ahead of time may improve the chance of success for this procedure. Such mobilization includes emergently summoning an obstetrician and, if available, a neonatologist to the bedside, as well as gathering an appropriate surgical tray, warmer or incubator, pediatric crash cart, and other necessary tools.96 Ideally, a trained obstetrician can perform the cesarean delivery while the emergency provider maintains attention on resuscitation of the mother. However, cesarean delivery is within the emergency physician’s scope of practice, and its performance should not be delayed to wait for a consultant.
To perform a perimortem cesarean delivery, a vertical incision is made through the skin and abdominal wall from the pubic symphysis to the xiphoid process. Time should not be wasted to decompress the bladder if not already performed, but decompressing the bladder in anticipation of possibly needing to perform cesarean delivery may lower the likelihood of inadvertent bladder injury. Once the incision has been made into the peritoneum, the uterus should be delivered anteriorly to allow better visualization.
Then, an incision is made into the uterus and, ideally, extended with scissors to decrease the chances of lacerating the fetus. For most pregnancies, the uterine incision should be made low because the placenta is more likely to be superior. If the placental position is already known, preferentially make the incision opposite to its location, but if the placenta is anterior, it should be cut through to maintain speed.
The fetus is delivered from the uterus, and the umbilical cord is clamped rapidly and cut. The fetus is handed off to an assistant for resuscitation. Manual delivery of the placenta is followed by packing the uterus. If the return of spontaneous circulation is achieved, manual pressure to the uterus may need to be applied to compensate for atony. Oxytocin can be administered, but it should be given slowly to avoid hypotensive side effects.97
Prevention
Seat Belt and Airbag Use
The use of properly positioned seat belts is associated with a substantial reduction in harm to pregnant patients involved in motor vehicle collisions. Unfortunately, less than half of all pregnant patients report consistent, proper use of seat belts.98 In one analysis of 57 motor vehicle collisions involving pregnant patients, researchers found that up to half of all cases of fetal demise could have been prevented with the proper use of seat belts.99 All prenatal programs should include education about the proper positioning of seat belts. Recommendations from the National Highway Traffic Safety Administration state that the shoulder harness portion of the seat belt should be positioned over the collarbone between the pregnant woman’s breasts.100 The lap belt portion should be placed as low as possible under the pregnant abdomen and across the hips, not above or across the abdomen.
Airbag deployment is a marker of the severity of the mechanism of trauma. There is an association of increased risk of fetal demise and placental abruption with airbag deployment, but the airbag itself has not been shown to be causative.101,102
Prevention of Falls
Falls are the second most common cause of trauma in pregnant women. Up to one-quarter of women will fall at least once during their pregnancy.103 The anatomic changes of pregnancy lead to an increased risk of falls. This is because of the patient’s unfamiliarity with increased weight, reduced muscle strength, loosening of supporting joints, and an alteration in the center of gravity due to the expansion of the gravid abdomen. The ability to control balance decreases as the patient approaches the middle of the second trimester.104 All prenatal programs should include fall prevention education and training for improved public health.
Domestic Violence Screening
Pregnant women are at disproportionately high risk for domestic or intimate partner violence. The incidence of violence increases throughout pregnancy, peaking in the third trimester.105 Women who have had counseling for intimate partner violence experienced fewer recurrent episodes of violence both during and after pregnancy, as well as better birth outcomes.106 Any suspicious injuries should prompt questioning about safety, and providers should make all efforts to talk to the patient alone.
For various reasons, some women may not feel comfortable seeking help or disclosing intimate partner violence during their emergency department evaluation. Domestic violence is almost assuredly underreported. If counseling and information are provided routinely by a multidisciplinary team when encountering the pregnant trauma patient, even patients who are not ready to disclose or take action at the time of the encounter will have resources available to them for when they are.107
Conclusion
Pregnant patients are a special population who require careful consideration of anatomic and physiologic changes, patterns of injury, and fetal assessment when being evaluated after trauma. Significant morbidity and mortality, including preterm delivery, are associated with traumatic injuries. It is important to remember that the best chance of a good outcome for the fetus is successful resuscitation of the mother, who should be prioritized throughout the evaluation. Multidisciplinary care, including early obstetrical consultation at the time of the secondary survey, is good practice. Injury prevention and education are important public health opportunities that could serve to reduce the overall impact of trauma in pregnancy.
REFERENCES
A complete list of references is available online: https://www.reliasmedia.com/ext/resources/newsletters/t/TR/2020/05/TR-050120-References.pdf
The authors provide a concise, comprehensive overview of the unique anatomic and physiologic features of pregnancy, as well as modifications and considerations important for the management of the pregnant trauma patient.
Subscribe Now for Access
You have reached your article limit for the month. We hope you found our articles both enjoyable and insightful. For information on new subscriptions, product trials, alternative billing arrangements or group and site discounts please call 800-688-2421. We look forward to having you as a long-term member of the Relias Media community.
