AUTHORS
Andrew J. Matuskowitz, MD, Chest Pain Center Medical Director, Medical University of South Carolina, Charleston
Hesham Abukhdeir, MD, Medical University of South Carolina, Charleston
Kyle Weant, PharmD, BCPS, FCCP, Medical University of South Carolina, Charleston
Chara Calhoun, PharmD, BCPS, Medical University of South Carolina, Charleston
Brant Domangue, MD, Medical University of South Carolina, Charleston
Jeffrey Caporossi, MD, Assistant Professor, Medical University of South Carolina, Charleston
PEER REVIEWER
Lance D. Wilson, MD, Department of Emergency Medicine, MetroHealth Campus, Case Western Reserve University, Cleveland, OH
EXECUTIVE SUMMARY
- The incidence of atrial fibrillation increases with age.
- Hypertension is the most common modifiable risk factor for atrial fibrillation.
- Rate or rhythm control approaches for patients with symptomatic atrial fibrillation yield equivalent short- and long-term clinical outcomes.
- Rhythm control in appropriate patients is associated with shorter ED lengths of stay and reduced hospital admission rates.
- All patients with atrial fibrillation should be evaluated for the need for anticoagulation to reduce the risk of stroke.
As an emergency physician, how do you manage acute onset atrial fibrillation (AF) at your institution? Do you instinctively reach for beta- and calcium channel blockers, as many of us in the United States have been trained, or do you aggressively cardiovert recent-onset AF with electricity, procainamide, or yet another antiarrhythmic agent? While both are safe, the literature suggests that the decision to pursue rate vs. rhythm control should depend on the specific characteristics of the patient.
The following article provides an overview of AF and evidence-based guidance on controversial aspects of AF workup and management in the emergency department (ED). The evidence is provided to help safely reduce unnecessary testing and expand the emergency provider’s management armamentarium to include electrical and pharmacologic conversion in recent-onset AF patients.Finally, the article integrates the evidence into a novel AF protocol, which in collaboration with cardiology and pharmacology stakeholders, may serve as a starting point for improving AF management at your institution.
Case Introductions
Case 1: A 45-year-old male presents to the ED with sudden onset palpitations and diaphoresis. Symptoms began suddenly five hours ago while at rest. He denies pain, describes the feeling as “uneasy” and constant since onset. The patient smokes 20 packs of cigarettes per year and drinks two to three beers per week but otherwise denies past medical history and has felt well until today. Triage vital signs are: 37.2°C, 110 beats/min, 122/75 mmHg, 21 breaths/min, SpO2 100% on room air. The physical exam reveals tachycardia, no jugular venous distention, clear lung sounds, and no peripheral edema. What is in your differential diagnosis? What tests and/or imaging should you order? What are your options for treating this patient? What is this patient’s disposition after treatment?
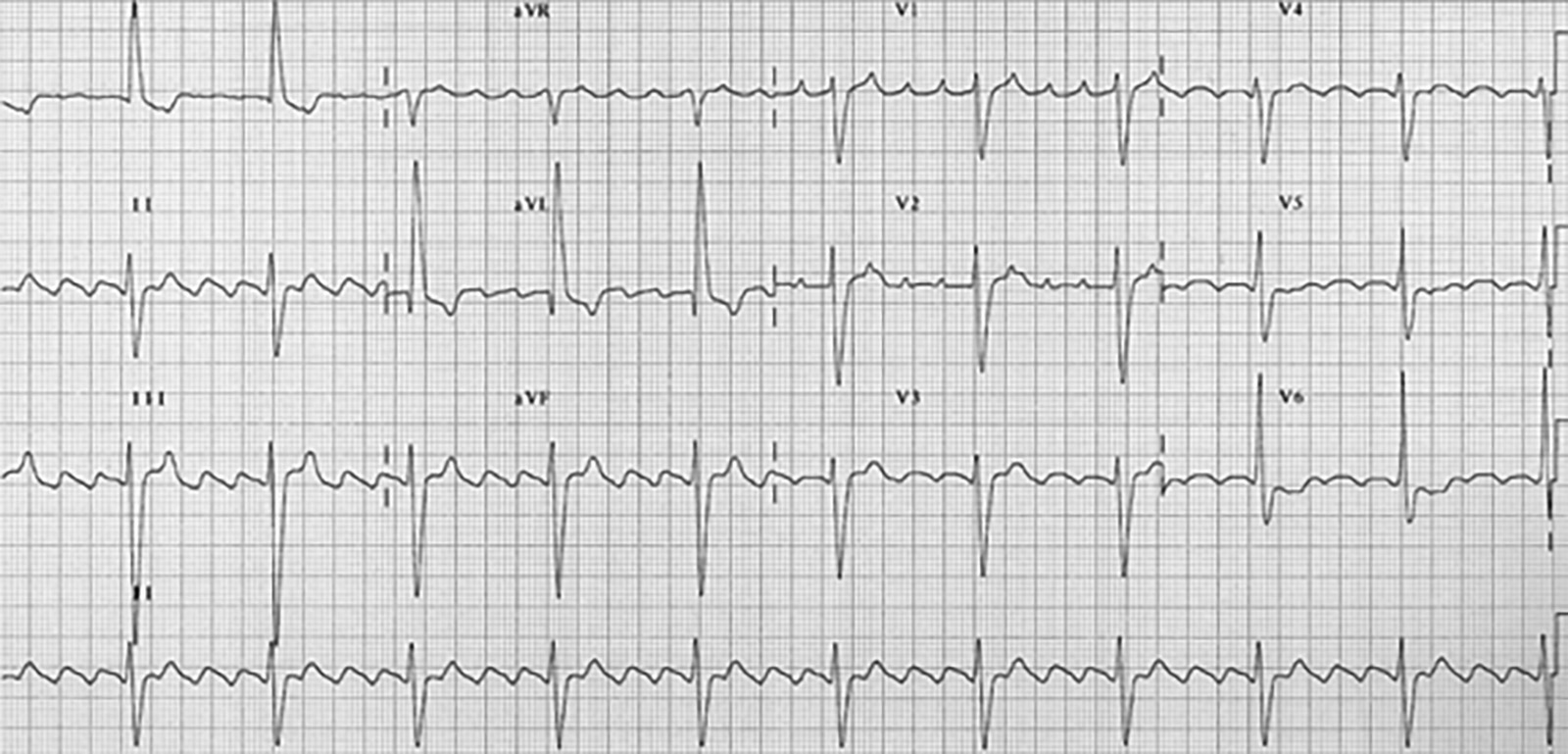
Case 2: A 68-year-old female presents with anxiety. She has associated palpitations, shortness of breath, and perioral tingling. Past medical history includes AF, well-controlled diabetes, and hypertension. Medications include sitagliptin, amlodipine, metoprolol, and apixaban. The patient was diagnosed with AF five years ago and has not felt her heart racing since she was placed on metoprolol and apixaban at that time. Triage vital signs: 37.0°C, 160 beats/min, 140/90 mmHg, 24 breaths/min, 94% on room air. Physical exam reveals irregular tachycardia, clear lung sounds, and no peripheral edema. Her electrocardiogram (ECG) is shown in Figure 1. What is on your differential diagnosis? What other questions need to be answered to treat this patient? What tests and/or imaging should you order? What are your options for treating this patient?
Figure 1. Case 2 ECG |
 |
|
Figure used with permission from William J. Brady, MD. |
Case 3: A 50-year-old male is brought in by EMS and appears acutely ill. Initially, the patient was alert and oriented with stable vital signs despite an irregular heart rhythm. However, upon arrival, he became diaphoretic and confused. The nurses have obtained IV access and placed the patient on the monitor. Vital signs are: 36.5°C, 270 beats/min, 85/40 mmHg, 12 breaths/min, 99% on 2 L NC. What are your first steps in managing this patient? What tests and/or imaging should you order? What is this patient’s disposition after treatment?
Definition and Relevancy
AF, the most common supraventricular tachyarrhythmia, can lead to stroke, heart failure, and cardiac arrest if untreated. AF results from the excitation of multiple atrial foci that create excessive and poorly coordinated atrial activation and contraction.1 ECG characteristics include “irregularly irregular” RR intervals without consistent or distinct P waves.1 Decreased efficiency of blood flow through the atria can lead to atrial thrombus formation and results in a four- to five-fold increased risk of embolic stroke.2 In fact, an estimated 20-30% of strokes may be secondary to AF.3 Sustained, uncontrolled AF with rapid ventricular response (RVR) over the course of weeks can lead to tachycardia-related cardiomyopathy. While uncommon, even short-lived AF with very fast ventricular rates can cause hemodynamic instability and cardiac arrest.4
Epidemiology and Risk Factors
The incidence of AF increases with age, and it affects approximately one in four people during their lifetime. There are roughly 3 million people with AF in the United States alone,5,6 and as life expectancy climbs, this number is expected to increase five-fold by 2050.7 AF has been estimated to have a total incremental healthcare cost of $8,705 per patient per year, approximating to a $26 billion healthcare burden in the United States.8 Consequently, cost-effective evidence-based management strategies are needed to limit escalating healthcare costs.
Many factors increase a patient’s risk for AF. Age, the most predominant risk factor, increases AF’s prevalence from 1% to 15% between the ages of 35 and 85 years.9 Men are slightly more likely to develop AF than women, but women tend to live longer, so they represent half of the overall AF population.10 Hypertension increases the risk of developing AF by more than 40%, and hypertension’s enormous prevalence in the general populations makes it the most important modifiable risk factor.11 Heart failure has a complex relationship with AF: The presence of one increases the likelihood of the other. As the severity of heart failure progresses, the prevalence of AF increases from < 5% to 50%.12 Other risk factors for AF include coronary artery disease, valvulopathies, obesity, sleep apnea, and hypertrophic cardiomyopathy.9
Classification and Pathophysiology
AF typically is classified as paroxysmal, persistent, or permanent.1,13 Paroxysmal AF is defined by atrial fibrillation that converts to sinus rhythm, either spontaneously or within seven days after intervention. Persistent AF lasts longer than seven days and usually requires cardioversion to convert to sinus rhythm. Permanent AF refers to individuals with persistent AF either refractory to cardioversion or where a decision has been made to no longer attempt to convert to sinus rhythm. New-onset AF refers to first-diagnosed or first-detected AF, and recent-onset refers to symptomatic AF that started within the last 48 hours. Finally, the term nonvalvular AF refers to atrial fibrillation in the absence of rheumatic disease, mitral stenosis, a prosthetic valve, or mitral valve repair.
Various events may incite AF, including autonomic nervous system stimulation, bradycardia, atrial premature beats, atrial tachycardia, accessory AV pathways, acute atrial stretch, and ectopic foci in atrial tissue.1,13,14 Repeated inciting events can lead to remodeling of the atria and the creation of multifocal reentrant loops, typically in the left atrium near the pulmonary veins.13,15 As inciting events accumulate and remodeling continues, spontaneously returning to sinus rhythm, maintaining sinus rhythm, and successful cardioversion become more difficult.13
Clinical Features
AF is characterized on the ECG by an irregularly irregular ventricular rate and an absence of discernable P waves. (See Figures 2-4.) There also may be fine or coarse fibrillation waves that can be mistaken for P waves. Often, symptomatic patients present with an RVR, defined by a rate > 100 beats per minute (bpm). Symptoms typically include palpitations, shortness of breath, dizziness, fatigue, anxiety, and chest discomfort. Signs typically include an irregular heartbeat on auscultation and an irregular pulse on palpation.
Figure 2. Atrial Fibrillation With Coarse Atrial Fibrillation Waves in Lead V1 |
 |
|
Figure used with permission from www.lifeinthefastlane.com. |
Figure 3. Atrial Fibrillation With Fine Atrial Fibrillation Waves in Lead V1 |
 |
|
Figure used with permission from www.lifeinthefastlane.com. |
Figure 4. Atrial Fibrillation With Rapid Ventricular Response |
 |
|
Figure used with permission from www.lifeinthefastlane.com. |
Diagnostic Testing
Typical Workup
Despite the impulse to immediately correct this abnormal rhythm, be wary that AF with RVR often occurs in response to other acute disease processes. For instance, initiating rate control therapy in a septic patient whose RVR is attempting to maintain adequate tissue perfusion could trigger hypotension or cardiovascular collapse.16 See Table 1 for a list of can’t-miss pathologies that may present with AF.
History, physical exam, and knowledge of potential AF triggers dictate further testing. Initially, vital signs, complete blood count (CBC), and basic metabolic panel (BMP) usually are the laboratory tests required. Generally, there is no indication for liver transaminases and coagulation studies unless directed by history and physical. If a pulmonary embolism or deep venous thrombosis is a consideration, then D-dimer, venous duplex ultrasound, and/or CT angiography of the chest may be required. If overdose is a possibility, then some combination of ethanol level, urine drug screen, acetaminophen level, salicylate level, etc., may be required. Chest X-ray may be helpful to identify signs of heart failure, heart size, or pulmonary pathology.
Table 1. Can’t-miss Pathologies That May Present With Atrial Fibrillation |
|
|
Disease Process |
History, Signs, and Symptoms |
|
Acute coronary syndrome (ACS) |
Chest pressure, diaphoresis, ST elevation on ECG, positive biomarkers, dyspnea on exertion |
|
Congestive heart failure (CHF) |
Shortness of breath, S3, crackles on lung exam, jugular vein distension, hepatojugular reflex, bilateral lower extremity edema, and chest X-ray with edema |
|
Pulmonary embolism (PE) |
Pleuritic chest pain, unilateral extremity swelling, malignancy, prior deep vein thrombosis/pulmonary embolism, recent surgery or trauma, hemoptysis, oxygen saturation < 95% |
|
Pericarditis/myocarditis |
Chest pain, friction rub, PR depression, ST elevations, recent myocardial infarction/virus, positive troponin |
|
Hyperthyroidism/thyroid storm |
Hypertension, brisk reflexes, tremors, weight loss with normal appetite, heat intolerance |
|
Valvular heart disease (e.g., mitral stenosis, regurgitation) |
New murmur, recent ACS, history of CHF, syncope |
|
Alcohol withdrawal |
Tremors, anxiety |
|
Sepsis |
Quick sepsis related organ failure assessment (qSOFA) or systemic inflammatory response syndrome (SIRS) criteria |
|
Drug overdose |
History of drug use, toxidrome features |
|
Gastrointestinal bleed |
Positive fecal occult blood test, liver disease, peptic ulcer disease, melena, hematochezia, hematemesis |
Limited Indications for Echocardiogram
Formal echocardiogram generally is not indicated in the emergency setting when there is low suspicion for concomitant structural or functional heart anomalies. Exceptions include patients for whom flecainide or propafenone are being considered as treatment options. These two Class IC antiarrhythmic medications should be administered only in patients with structurally normal cardiac function, as discussed in the “Choosing the Right Rhythm Control Agent” section.3,4,17 A transthoracic echocardiogram (TTE) can evaluate cardiac structure and function in these patients and is available in many EDs. A transesophageal echocardiogram (TEE) is required when a rhythm control strategy is being considered and duration of AF is unknown. Only TEE — not TTE — is sensitive enough to rule out atrial thrombi, but TEE requires conscious sedation and typically an inpatient admission.
Limited Indications for Thyroid Stimulating Hormone Testing
Perhaps relatively low cost and decades of entrenched behavior have led many providers to order a thyroid-stimulating hormone (TSH) test indiscriminately for patients in AF with RVR. Evidence suggests that this approach is overkill. One retrospective study of nearly 2,000 patients with AF found low TSH indicative of hyperthyroidism in only 2% of patients, most commonly in those with new-onset AF or prior history of hyperthyroidism.18 Other studies have reported similarly low yields of detecting thyroid disease.19-21 Therefore, perform TSH testing only in patients with new-onset AF or hyperthyroidism for whom TSH testing has not occurred within the past three months.
Limited Indications for Troponin Testing and Importance of ST Segment Changes
Suspicion for acute coronary syndrome (ACS) causing or accompanying AF is an indication for cardiac biomarkers, but routine testing is unnecessary.Three studies investigating the causes of elevated troponin levels in the setting of tachyarrhythmias, including AF, found that troponin elevation was significantly more commonly associated with the rapid heart rate than ACS.22-24 They concluded that elevated troponins in patients without definite clinical or electrocardiographic evidence of myocardial ischemia is not predictive of cardiovascular complications or death at one year.
ST segment elevations in concordant leads on any ECG should be evaluated for acute myocardial infarction (MI).25,26 However, contrary to popular belief, transient ST segment depression during AF with RVR is not equivalent to a positive stress test. In one prospective cohort study in which 109 patients with AF and symptoms suggestive of ACS underwent a rule out MI protocol, only six (5.5%) suffered an MI.26 The six patients with MI were more likely to have chest pain, new-onset AF, ST segment elevation, or ST segment depression > 2 mm than the 103 patients who did not have infarction. Either major ST segment depression (> 2 mm) or any ST segment elevation on the admission ECG had a sensitivity of 100% and a specificity of 99% for MI. Other studies similarly found that transient ST segment depression does not correlate with underlying coronary artery disease (CAD).27,28 Therefore, in general for patients presenting with AF, ACS should be evaluated only when the patient specifically complains of chest discomfort or there is ST segment elevation or
> 2 mm ST segment depression on the ECG.
Differential Diagnosis
AF can be mistaken for other arrhythmias, especially supraventricular tachycardia (SVT) and atrial flutter (AFL). SVT includes sinus tachycardia, atrioventricular nodal re-entry tachycardia (AVNRT), and atrioventricular reciprocating tachycardia (AVRT), all of which demonstrate regular RR intervals. Flutter waves, found in AFL, are characterized by large, inverted “saw tooth” P waves, typically in the inferior leads. In AFL, a re-entry circuit creates atrial impulses typically at a rate of 300 with varying degree of transmission through the atrioventricular (AV) node, resulting in 1:1, 2:1, 3:1, 4:1, or variable conduction ratios. (See Figure 5.)
Figure 5. Atrial Flutter |
 |
|
Atrial flutter with 4:1 AV conduction. Note the deep, “saw-tooth” inverted P waves in leads II, III, and aVF. Figure used with permission from www.lifeinthefastlane.com. |
Distinguishing AF from other types of tachycardia becomes increasingly difficult as heart rate increases. In cases of extreme SVT where AVNRT, AVRT, and AFL are at least as likely as AF, a transient AV node block via vagal maneuver or intravenous adenosine may help distinguish between or even resolve the tachyarrhythmia.
Special Consideration: Wolff-Parkinson-White Syndrome With Atrial Fibrillation
Wolff-Parkinson-White syndrome (WPW) with AF merits discussion because mistaking it for AF with RVR can have devastating consequences. WPW is characterized by an accessory pathway through which atrial impulses can bypass the AV node. ECG features of WPW during sinus rhythm include shortened PR-interval, slurring of the initial portion of the QRS complex (delta wave), slightly widened QRS complex, and T-wave inversions. (See Figure 6.) The most common tachyarrhythmia encountered in WPW is AVRT, which usually can be treated with vagal maneuvers or AV nodal blocking agents. However, administering AV nodal blocking agents in WPW with AF may promote unrestricted conduction through the accessory pathway, which can lead to rapid cardiovascular collapse.29
Figure 6. Wolff-Parkinson-White Syndrome in Normal Sinus Rhythm |
 |
|
Note the shortened PR-interval, delta wave, and slightly widened QRS complex. Figure used with permission from www.lifeinthefastlane.com. |
Although WPW is uncommon (< 0.3% of the population), AF comprises up to 40% of WPW tachyarrythmias.29 ECG characteristics of AF in WPW include wide, bizarre QRS complexes with significant beat-to-beat variations and variance in QRS width and amplitude, and may include slow QRS upstrokes — similar to delta waves — in the precordial leads. (See Figure 7.) Diagnosis of WPW with AF should be suspected in any patient with wide complex AF on ECG. ECGs of patients in AF with RVR and concomitant bundle branch blocks can be indistinguishable from WPW with AF, so reviewing prior ECGs is critical. Patients with WPW AF tend to be younger (usually < 50 years of age) with previous history of palpitations, syncope, or known WPW. Treatment of AF with WPW includes synchronized electrical cardioversion for unstable patients. If stable, chemical cardioversion may be attempted, provided electrical cardioversion is accessible immediately if the patient deteriorates. Procainamide often is considered the agent of choice, but both procainamide and ibutilide are reasonable.29,30 Ibutilide confers a slightly higher risk of developing torsades de pointes, while procainamide causes hypotension more frequently. Amiodarone should be used with caution in WPW with AF, as degeneration into ventricular fibrillation can occur.29
Figure 7. Wolff-Parkinson-White Syndrome With Atrial Fibrillation |
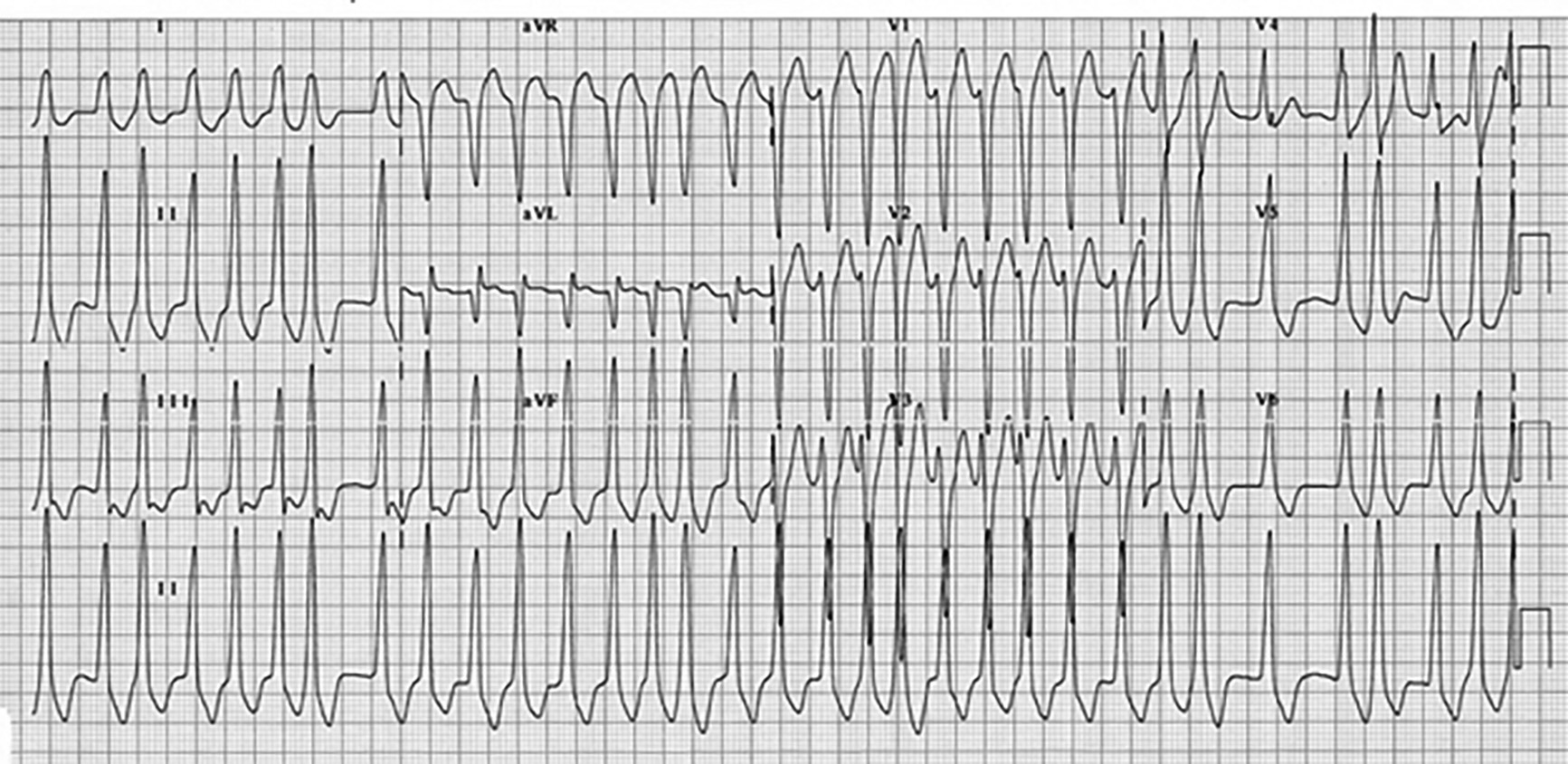 |
|
Note the irregular, widened QRS complexes and varying amplitude and width of the QRS complexes. Figure used with permission from www.lifeinthefastlane.com. |
ED Management
Standard Care for Every Patient With Atrial Fibrillation
In the acute setting, AF management focuses on stabilization, rate or rhythm control, and consideration for long-term anticoagulation therapy. AF requires an acute intervention when a patient is symptomatic, unstable, or demonstrates RVR.31,32 All patients who are symptomatic, unstable, or have AF with RVR require a cardiac monitor, oxygen if hypoxic, appropriate IV access, frequent blood pressure monitoring, and application of defibrillation pads. Rapid sequence intubation equipment also should be available at the bedside in case the patient’s clinical status deteriorates.
The Unstable Patient: Emergently Cardiovert and Consider Anticoagulation
In patients with signs of hemodynamic instability, including active myocardial ischemia, acute heart failure, or significant hypotension attributable to RVR, perform immediate synchronized electrical cardioversion. Consider sedation/analgesia medications, but do not delay cardioversion in an unstable patient.
Electrodes should be placed in the anterior-posterior (AP) or anterior-lateral (AL) positions; both are acceptable, but evidence suggests a slightly higher cardioversion success rate in the AP position.33,34 For unstable patients, the Advanced Cardiac Life Support (ACLS) guidelines recommend immediately administering synchronized electrical cardioversion using biphasic direct current at 120-200 Joules (J).35 The Canadian Cardiology Society (CCS) guidelines recommend starting at 150-200 J to increase likelihood of initial success.4 For obese patients, consider administering 200 J; one randomized controlled study found that in patients with a BMI greater than 25, there was a 31% higher successful first-shock rate with 200 J compared to starting at a lower dose.36
Unstable patients with known duration greater than 48 hours, unknown duration, or an increased stroke risk additionally should receive a full venous thromboembolism dose of heparin before cardioversion or immediately afterward provided anticoagulation is not contraindicated. Such a patient should continue with anticoagulation for four weeks or longer post-cardioversion to reduce stroke risk.4,37
The Stable Patient
In a hemodynamically stable patient with symptomatic AF or RVR, rate or rhythm control should be initiated. While it is intuitive that restoring normal sinus rhythm would improve long-term outcomes, numerous studies have demonstrated no differences between rate and rhythm control in long-term risk of stroke, death, or any other major adverse outcome.38-40 Therefore, the decision to pursue rate vs. rhythm control in the acute, stable patient depends on comorbidities, time of symptom onset, and shared decision-making with the patient.
Indications for Rate Control
Rate control is safe for most stable patients regardless of onset time. Rate control also should be used in patients with permanent AF, older patients with persistent AF, symptom onset greater than 48 hours or of unknown duration, recent transient ischemic attack (TIA)/stroke, and valvular disease.4,16
Rate Control: Metoprolol vs. Diltiazem
Both beta-blockers and calcium channel blocking agents slow AV conduction and prolong AV node refractoriness in acute AF. Metoprolol is the beta-blocker of choice because it selectively blocks beta-1 receptors in the heart, whereas nonselective agents also act on beta-2 receptors, increasing the risk of hypotension. Metoprolol is available in intravenous and oral formulations, making it an ideal rate-control agent while providing an easy transition to outpatient oral therapy.1,4,41 In non-decompensated heart failure, a beta-blocker is the drug of choice because of the potential negative inotropic effect of calcium channel blockers.1,3,4,41,42 Additionally, beta-blockers are the drugs of choice in the setting of AF secondary to an acute adrenergic surge or hyperthyroidism.
Although two classes of calcium channel blockers exist, only non-dihydropyridines calcium channel blockers (e.g., diltiazem, verapamil) are appropriate in symptomatic AF because of their negative chronotropic action. Like metoprolol, diltiazem is the drug of choice because of a wealth of supporting literature, decreased risk of negative inotropic and vasodilatory action, and availability as an oral and intravenous formulation.1,3,4,41
One study that randomized patients to receive diltiazem or metoprolol in patients with AF found that diltiazem was nearly 40% more successful at achieving a heart rate less than 100 bpm within five minutes than metoprolol, and approximately 50% more successful within 30 minutes. Adverse effects occurred equally in each group.43 Therefore, while both agents are acceptable, diltiazem may be a more effective therapy in patients without heart failure.
Caution must be exercised when switching between IV beta- and calcium channel blockers. Several smaller studies have demonstrated symptomatic bradycardia and prolonged hypotension when these agents are combined.44,45 Thus, it may be more prudent to initiate multiple doses of the same agent to achieve rate control rather than switching between beta- and calcium channel blockers. See Table 2 for a rate control medication summary.
Table 2. Rate Control Agent Summary |
||||
|
Primary Rate Control Agents |
||||
|
Drug |
Acute Treatment in ED |
Home Regimen |
Side Effects |
Comments |
|
Metoprolol |
5 mg IV every 5 minutes until target heart rate achieved (max 15 mg) |
Metoprolol tartrate (immediate release) OR Metoprolol succinate (extended release) |
• Bradycardia, hypotension, potential activation of reactive airway disease |
• If current home medication, increase to next available dosage form • Metoprolol tartrate less expensive than succinate form • Preferred in non-decompensated HF |
|
Diltiazem |
0.25 mg/kg IV once followed by a repeat dose of 0.35 mg/kg if target HR not achieved |
Diltiazem IR 30 mg PO every six hours OR Diltiazem CD/LA/ER/XT 120 mg PO daily |
• Bradycardia, hypotension • Avoid in heart failure |
• If current home medication, increase to next available dosage form • Generally more expensive than metoprolol tartrate |
Rhythm Control: Advantages in Recent-onset Atrial Fibrillation
While rate control is a time-honored, conservative approach to managing patients with symptomatic AF in the ED, rhythm control may have significant advantages when used in certain populations. Overcrowding, lack of hospital beds, and prolonged ED lengths of stay are common in many North American hospitals.46 Many Canadian and a few U.S. institutions, therefore, have adopted a more aggressive rhythm control strategy, which has led to shorter ED stays and reduced admission rates. In the United States, approximately 30% of patients presenting with AF are discharged, but in Canada more than 60% are discharged.47-49
Rhythm control is ideal for patients with paroxysmal AF, especially those with new or recent-onset AF (less than 48 hours) or on anticoagulation for at least three weeks.4,17 Rhythm control should be avoided if symptom onset is unknown, and patient-reported history is critical to determining symptom onset. Some clinicians feel uncomfortable relying on patient self-reported symptom onset. This concern has some merit. Two studies suggest that 35-37% of patients in AF do not perceive symptoms, although patients in these studies tended to be > 65 years old and had implantable cardiac devices suggesting diseased hearts at baseline.50,51 In contrast, emergency medicine literature consistently supports that when known, patients’ self-reporting of symptom onset is accurate and reliable.46,52,53 When ED patients report < 48 hours of symptoms, rhythm control confers < 1% risk of stroke or death in the short- and long-term.46,49,53
History also is important in determining anticoagulation compliance. Patients on any of the direct oral anticoagulants (DOACs) need to be compliant on their medication for at least three weeks prior to attempting rhythm control.37 If the patient is on warfarin, INR should be obtained first to determine if the patient is appropriately anticoagulated. Finally, rhythm control should be avoided in patients with permanent AF, older patients with persistent AF, symptom onset greater than 48 hours or of unknown duration, recent TIA/stroke, and valvular disease. See Table 3 for characteristics that favor rate vs. rhythm control.
Table 3. Characteristics Favoring Rate vs. Rhythm Control |
|
|
Characteristics Favoring Rate Control |
Characteristics Favoring Rhythm Control |
|
• Age > 65 • Many comorbidities • Symptom onset > 48 hours or unknown • Longstanding or persistent AF • Recent TIA or stroke • Rheumatic heart disease • Mechanic heart valve |
• Age < 65 • Few comorbidities • Symptom onset < 48 hours • Paroxysmal AF • Anticoagulated for ≥ 3 weeks or therapeutic INR |
Rhythm Control: Electrical vs. Pharmacologic Cardioversion
Choosing between electrical and pharmacologic cardioversion to restore sinus rhythm depends on the patient’s blood pressure, risks of sedation, and patient and provider preference. There are some clear benefits to electrical compared to pharmacologic cardioversion. First, electrical cardioversion boasts significantly higher success rates of 89-96% compared to pharmacologic cardioversion success rates of 50-83%.4,46,53,54 Second, once the patient is sedated, electrical cardioversion is instantaneous, whereas pharmacologic cardioversion may take several hours depending on the agent. Third, several antiarrhythmic agents cannot be used in patients with structural or functional heart disease, and none of them are safe in patients with low blood pressures.
Despite these advantages, the pharmacologic approach has an important role as well. When successful, rhythm control medications eliminate the need for procedural sedation and associated risks. Also, many hemodynamically stable patients are uncomfortable with the notion of electrical shock when another option is available. Therefore, clinicians should become comfortable with using several of the various antiarrhythmic agents. Note, pretreatment with rate control agents has not been shown to enhance the rate of cardioversion using antiarrythmics.4
Choosing the Right Rhythm Control Agent
The most commonly used antiarrhythmics include Class IA, IC, and III agents. Each has a unique set of advantages and disadvantages that must be considered based on the comorbidities of the patient.
Procainamide, a Class IA antiarrhythmic, is an increasingly attractive option for rhythm control and has been used preferentially and safely in many Canadian EDs for years. A sodium channel blocking agent, procainamide has a 58-65% cardioversion rate for AF, often within one hour.46,52,55 It boasts fewer side effects than Class IC agents, the most common being transient hypotension in 5-8% of cases. Unlike Class IC antiarrhythmics, procainamide can be used in patients with stable structural and functional heart disease.4,54,56 In cases in which procainamide fails to restore normal sinus rhythm within one to two hours, electrical cardioversion can be initiated safely.4
For patients with paroxysmal AF, flecainide and propafenone are two of the most commonly prescribed antiarrhyhmics in the outpatient setting by cardiologists. Their use in the acute setting, however, remains less clear. Both are Class IC agents that block sodium channels, slow conduction through the heart, and exert a negative inotropic effect.57 Acute treatment with flecainide is associated with a conversion rate of 51% at three hours and 72% at eight hours.58 Similarly, propafenone effectively cardioverts patients 56-83% of the time, typically within three to four hours.59,60 Monitoring patients for this length of time may not be tenable in many busy EDs. Additionally, these agents cannot be used in patients with structural or functional heart disease, including left ventricular dysfunction, heart failure, sick sinus syndrome, bundle branch blocks, or AV blocks.37 Therefore, an echocardiogram should be obtained prior to administering these agents if not recently performed.
Ibutilide and amiodarone are Class III antiarrhythmics that primarily prolong action potential in cardiac tissue. The excessive cost and the lower rate of cardioversion makes ibutilide less favorable than procainamide. Ibutilide has demonstrated conversation rates of 40% at 90 minutes.61 Furthermore, it is recommended that patients receiving ibutilide be monitored for at least four hours after administration, making its use in the ED cumbersome.41,62-64 Amiodarone rarely is used in recent-onset AF because of significantly lower conversion rates and higher side effect profiles than the aforementioned agents.65-67 However, amiodarone can be used in patients with acute ischemia, acute MI, and LV dysfunction.68 See Table 4 for a rhythm control medication summary.
Table 4. Rhythm Control Agent Summary |
||||||
|
Drug |
Class |
Mechanism |
Dose |
Conversion Rate |
Comments |
Adverse and Side Effects |
|
Procainamide |
IA |
• Blocks sodium channels |
• 15-17 mg/kg IV over 60 min • Alternatively, 1,000 mg IV over 30 min |
• 58.3% at 1 hr |
• Safer in structural heart disease compared to Class IC agents |
• Hypotension rate 5-8.5% • Bradycardia • Torsades de pointes |
|
Propafenone |
IC |
• Blocks sodium channels • Weakly blocks potassium/calcium channels and beta receptors |
• < 70 kg: 450 mg PO • > 70 kg: 600 mg PO |
• 56-83%, typically within 2-3 hrs • Success dependent on AF duration |
• Exclude in left ventricular dysfunction or heart failure, sick sinus syndrome, QRS duration ≥ 110 msec, and second- or third-degree AV block |
• Transient arrhythmias and hypotension • Reversible QRS widening • Nausea • Metallic taste in mouth |
|
Flecainide |
IC |
• Blocks sodium channels and slows conduction through the heart • Transiently decreases cardiac output and stroke volume |
• < 70 kg: 200 mg PO • > 70 kg: 300 mg PO |
• 51-72% within 8 hrs, typically within 3-5 hrs |
• Great oral bioavailability • Minimal effects on blood pressure • Exclusions similar to propafenone |
• Headache • Dizziness • Visual disturbances • Tremor |
|
Ibutilide |
III |
• Prolongs action potential in cardiac tissue |
• < 60 kg: 0.01 mg/kg IV over 10 min • > 60 kg: 1 mg IV over 10 min |
• 27-40% in 60-90 min |
• Some data suggest pretreatment with magnesium is warranted • Expensive • ECG monitoring for 4 hours afterward |
• Hypotension • Bradycardia • Torsades de pointes (3%) risk |
|
Amiodarone |
III |
• Prolongs phase 3 of cardiac action potential • Acts on multiple channels and nonselective inhibition of alpha and beta receptors |
• Bolus of 5 mg/kg IV followed by an infusion of 1.2-1.8 gm over 24 hrs • 150 mg in 10 min followed by 1 mg/min for 6 hrs then 0.5 mg/min for 18 hrs |
• Failed to show efficacy for the acute treatment of recent-onset AF |
• Drug of choice for managing AF in setting of acute ischemia, acute MI, or LV dysfunction • Safe in structural heart disease • Not FDA approved for atrial fibrillation |
• IV: phlebitis, hypotension • Chronic toxicities: - Photosensitivity - Hyperthyroidism - Hypothyroidism - Pulmonary fibrosis - Hepatotoxicty |
A Word on Digoxin and Magnesium
Digoxin, once the preferred agent in acute AF management, has fallen out of favor because of its delayed onset of action (up to six hours), complex impact on patients with heart failure, and poor cardioversion efficacy.69-71 Its use may be best limited to patients with hypotension and heart failure, ideally in consultation with a cardiologist.1,3,4,41
While not a traditional rate control agent, magnesium is recommended as an adjunctive agent for AF patients in the acute setting because it may decrease conduction through the AV node and intra-arterial conduction times.72,73 Many patients with arrhythmias have been shown to have intracellular magnesium deficiency.74,75 Prophylactic magnesium use with ibutilide for chemical cardioversion has shown increased conversion rates and a decreased incidence of side effects from ibutilide.75-77
Disposition Considerations
Indications for admission of patients with AF include hemodynamic instability, serious alternative primary diseases, diagnostic uncertainty, and failure of symptomatic, rate, or rhythm control. Because of the risk of tachycardia-induced cardiomyopathy, a patient who remains in AF at a rate greater than 100 bpm at rest and 110 bpm with ambulation should not be discharged.4,17 Even when rate or rhythm is well controlled but comorbidities abound, appropriate disposition may be unclear.
One clinical decision support tool that has shown promise in determining which patients are safe for discharge is the Risk Estimator Decision Aid for AF (RED-AF).78 A validation study of RED-AF has demonstrated that low-risk patients have less than 7% chance of having an adverse event including ED re-visits, rehospitalizations, cardiovascular complications, and death.79
Stroke Risk: Always Calculate CHA2DS2-VASc
Because patients with AF have an inherently increased stroke risk, estimating the patient-specific stroke risk is essential prior to discharge. That risk of stroke can be reduced by oral anticoagulation, but with an increased risk for bleeding. The challenge has been to identify patients for whom long-term anticoagulant therapy can reduce the risk of stroke with an acceptable risk for hemorrhage. The preferred risk-stratifying tool in patients with nonvalvular AF is the CHA2DS2-VASc score.37,81,82 See Tables 5 and 6 for CHA2DS2-VASc score calculation and recommendations for long-term anticoagulation in patients with AF, respectively.
If anticoagulation is necessary, the choice is between a low molecular-weight heparin bridge with warfarin or one of the newer DOACs. Each DOAC is superior to or as effective in reducing stroke as warfarin in nonvalvular AF. In fact, all DOACs demonstrated lower rates of intracranial hemorrhage (ICH), the most feared bleeding complication, and lower or similar rates of fatal and major bleeds.83 Moreover, DOACs do not require INR monitoring, a not-to-be overlooked benefit to the patient.
Table 5. CHA2DS2-VASc Score Calculation |
||
|
|
Condition |
Points |
|
C |
Congestive heart failure (or left ventricular systolic dysfunction) |
1 |
|
H |
Hypertension: Blood pressure consistently above 140/90 mmHg (or treated hypertension on medication) |
1 |
|
A2 |
Age ≥ 75 years |
2 |
|
D |
Diabetes mellitus |
1 |
|
S2 |
Prior stroke, transient ischemic attack, or thromboembolism |
2 |
|
V |
Vascular disease (e.g., peripheral artery disease, myocardial infarction, aortic plaque) |
1 |
|
A |
Age 65-74 years |
1 |
|
Sc |
Sex category (i.e., female) |
1 |
Table 6. Recommendations for Long-term Anticoagulation in Patients With Atrial Fibrillation |
||
|
CHA2DS2-VASc Score |
Adjusted Stroke Rate |
Start Anticoagulation? |
|
0 |
< 1% |
No |
|
1 |
1.3% |
Consider aspirin vs. anticoagulation |
|
≥ 2 |
2.2% |
Yes |
While all DOACs effectively prevent stroke, compliance and cost are important considerations. For instance, it may be much easier for patients to remember to take medications that require once-daily dosing compared to twice-daily dosing. Additionally, it is a disservice to the patient to prescribe a medication that he or she cannot afford. For instance, the authors of this article inquired into the cost of one of the most commonly used DOACs and learned that even with Medicare coverage, the monthly cost without additional coverage benefits approaches $300 monthly, an unaffordable price for many Americans. Fortunately, all DOACs have patient assistance or prescription vouchers available through the manufacturers. ED and inpatient pharmacists are great resources for this assistance. See Table 7 for a comparison of DOAC agents.
Table 7. Comparison of Direct Oral Anticoagulants |
||||
|
Agent |
Mechanism of Action |
Dose |
Dose Adjustments |
Trials Comparing Direct Oral Anticoagulants to Warfarin |
|
Dabigatran |
Direct thrombin inhibitor |
150 mg twice daily |
Reduce dose to 75 mg BID if creatinine clearance (CrCl) 15-30 mL/min. No recommendations if CrCl < 15 mL/min or on dialysis |
RE-LY: Lower rate of ICH and major GI bleeds; no reduction in major or fatal bleeds |
|
Rivaroxaban |
Factor Xa inhibition |
20 mg daily with evening meal |
Reduce dose to 15 mg once daily for CrCl 15-50 mL/min |
ROCKET AF: Lower rate of ICH, fatal and major GI bleeds; no reduction in major bleeds |
|
Apixaban |
Factor Xa inhibition |
5 mg twice daily |
Serum creatinine ≥ 1.5 mg/dL and body weight ≤ 60 kg or age ≥ 80 years: Reduce dose to 2.5 mg twice daily |
ARISTOTLE: Lower rate of ICH and major bleeds; no reduction in major GI bleeding |
|
Edoxaban |
Factor Xa inhibition |
60 mg once daily |
Reduce dose to 30 mg once daily if CrCl is 15-50 mL/min; CrCl < 15 mL/min or > 95 mL/min: not recommended |
ENGAGE AF-TIMI: Lower rate of ICH, major, fatal, and major GI bleeds |
Outpatient Medication Recommendations: Keep It Simple
When rhythm control agents are used in the acute setting, prescribing rate control agents in the outpatient setting may provide distinct advantages and more optimal side-effect profiles. For new or recent-onset AF, consider prescribing a beta- or a calcium channel blocker (depending on the patient’s history), or simply increase the patient’s baseline home dose. If initiating a new medication upon ED discharge, provide adequate counseling, including monitoring parameters and possible adverse effects, and ensure patients have an opportunity to ask questions. When increasing the dose of a patient’s existing medication, it is recommended to provide a new prescription with the updated dosing regimen to ensure the patient takes the correct amount. See Figure 8 for medication recommendations in the outpatient setting.
Summary
Workup and management of AF depends on the patient presentation. TSH testing is not indicated except in new-onset AF or in a patient with hyperthyroidism who has not had TSH checked recently. Troponin testing generally is not indicated unless the patient endorses angina symptoms. ST segment elevation and ST segment depression > 2 mm in AF with RVR are concerning for ACS, but transient ST segment depression < 2 mm is not.
Unstable patients require immediate synchronized cardioversion, and anticoagulation should be administered if symptom onset is greater than 48 hours or of unknown duration, or if the patient has an increased stroke risk. Stable patients who reliably can confirm AF onset time of less than 48 hours, or those who have been anticoagulated consistently for three weeks can safely be cardioverted pharmacologically or electrically. Rate control also is reasonable in these patients provided that a slower rate improves their symptoms.
For patients with an alternative primary diagnosis, such as sepsis or acute decompensated heart failure, treat the primary diagnosis first; treating AF in these cases with rate control or cardioversion either is unlikely to be successful or could worsen clinical status.
In patients who are appropriate for discharge, calculating the CHA2DS2-VASc score determines whether long-term anticoagulation is necessary. Newer DOACs are at least as safe as warfarin and do not require monitoring. However, because of their high cost, ask the inpatient or ED pharmacists to contact the manufacturers to secure free vouchers and set up long-term plans to ensure patients can access these potentially lifesaving medications. See Figure 9 for an evidence-based suggested AF workflow.
Case Conclusions
Case 1: Differential Diagnosis: AF with RVR, AFL, SVT, PE, and hyperthyroidism
Tests/Imaging: CBC, BMP, and TSH (since new-onset AF). The ECG confirms AF with RVR.
Treatment options include rate control or rhythm control.
A CHA2DS2-VASc score should be calculated to determine the need for anticoagulation. The patient should have a follow-up appointment with a cardiologist and an outpatient echocardiogram.
Case 2: Differential Diagnosis: AF with RVR, AFL, SVT, and PE. This patient’s symptoms and ECG suggest low likelihood of ACS. Consider PE if shortness of breath does not resolve with intervention.
Important questions to ask: 1) Has she missed any apixaban doses in the past three weeks? 2) Does she remember the exact onset time?
Tests/Imaging: ECG, BMP, and CBC. ECG confirms the diagnosis of AF with RVR.
Treatment: If the patient has been taking apixaban consistently for at least three weeks without missing doses, and if she is confident that her AF started within the past 48 hours, then either rhythm or rate control are appropriate options. If she reports that she has missed doses in the past three weeks or is not certain of onset time, then her only option is rate control. If stable for discharge, then she should follow up with her cardiologist. Either her beta- or calcium channel blocker should be increased if possible.
Case 3: This patient is hemodynamically unstable and requires immediate synchronized electrical cardioversion. Place defibrillator pads on the patient in the anterior-posterior positions and obtain IV access if possible, but do not delay cardioversion. The patient should receive at least 120 J and up to 200 J if obese.
Once the patient is stabilized, a thorough history should be obtained regarding symptom onset, recent medical history, possible AF triggers, and past medical history. His answers will dictate the appropriate tests to order. If he has no history of hyperthyroidism and no prior symptoms concerning for ACS, then TSH and troponins should not be ordered. If the patient remains stable after cardioversion and workup, then discharge home may be reasonable. He should be started on a rate control agent or increased dose if he is already taking a rate control agent. A CHA2DS2-VASc score should be calculated to determine the need for long-term anticoagulation. The patient should have a follow-up appointment with cardiology and an outpatient echocardiogram if he has not had one recently.
REFERENCES
- January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2014;64:e1-76.
- Kanne WB, Wolf PA, Benjamin EJ, Levy D. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: Population-based estimates. Am J Cardiol 1998;82:2N-9N.
- Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J 2016;37:2893-2962.
- Stiell IG, Macle L; CCS Atrial Fibrillation Guidelines Committee. Canadian Cardiovascular Society atrial fibrillation guidelines 2010: Management of recent-onset atrial fibrillation and flutter in the emergency department. Can J Cardiol 2011;27:38-46.
- Chugh SS, Havmoeller R, Narayanan K, et al. Worldwide epidemiology of atrial fibrillation: A Global Burden of Disease 2010 Study. Circulation 2014;129:837-847.
- Naccarelli GV, Varker H, Lin J, Schulman KL. Increasing prevalence of atrial fibrillation and flutter in the United States. Am J Cardiol 2009;104:1534-1539.
- Miyasaka Y, Barnes ME, Gersh BJ, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation 2006;114:119-125.
- Kim MH, Johnston SS, Chu BC, et al. Estimation of total incremental health care costs in patients with atrial fibrillation in the United States. Circ Cardiovasc Qual Outcomes 2011;4:313-320.
- Andrade J, Khairy P, Dobrev D, Nattel S. The clinical profile and pathophysiology of atrial fibrillation: Relationships among clinical features, epidemiology, and mechanisms. Circ Res 2014;114:1453-1468.
- Anumonwo JM, Kalifa J. Risk factors and genetics of atrial fibrillation. Cardiol Clin 2014;32:485-494.
- Krahn AD, Manfreda J, Tate RB, et al. The natural history of atrial fibrillation: Incidence, risk factors, and prognosis in the Manitoba Follow-Up Study. Am J Med 1995;98:476-484.
- Maisel WH, Stevenson LW. Atrial fibrillation in heart failure: Epidemiology, pathophysiology, and rationale for therapy. Am J Cardiol 2003;91:2D-8D.
- Allessie MA, Boyden PA, Camm AJ, et al. Pathophysiology and prevention of atrial fibrillation. Circulation 2001;103:769-777.
- Lilly LS, Harvard Medical School. Pathophysiology of Heart Disease: A Collaborative Project of Medical Students and Faculty, 6th ed. Philadelphia: Wolters Kluwer; 2016: xi, 467.
- Brown J, Mazel J, Myerson S, et al. Cardiology Emergencies. Oxford University Press: New York; 2011: xii, 292.
- Vieillard-Baron A, Boyd J. Non-antiarrhythmic interventions in new onset and paroxysmal sepsis-related atrial fibrillation. Intensive Care Med 2018;44:94-97.
- Fuster V, Ryden LE, Cannom DS, et al. ACC/AHA/ESC 2006 Guidelines for the Management of Patients with Atrial Fibrillation: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation): Developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation 2006;114:e257-354.
- Bellew SD, Moman R, Lohse CM, et al. Validation of a decision rule for selective TSH screening in atrial fibrillation. West J Emerg Med 2015;16:195-202.
- Buccelletti F, et al. Utility of routine thyroid-stimulating hormone determination in new-onset atrial fibrillation in the ED. Am J Emerg Med 2011;29:1158-1162.
- Krahn AD, et al. How useful is thyroid function testing in patients with recent-onset atrial fibrillation? The Canadian Registry of Atrial Fibrillation Investigators. Arch Intern Med 1996;156:2221-2224.
- Gammage MD, Parle JV, Holder RL, et al. Association between serum free thyroxine concentration and atrial fibrillation. Arch Intern Med 2007;167:928-934.
- Redfearn DP, Ratib K, Marshall HJ, Griffith MJ. Supraventricular tachycardia promotes release of troponin I in patients with normal coronary arteries. Int J Cardiol 2005;102:521-522.
- Kanjwal K, Imran N, Grubb B, Kanjwal Y. Troponin elevation in patients with various tachycardias and normal epicardial coronaries. Indian Pacing Electrophysiol J 2008;8:172-174.
- Meshkat N, Austin E, Moineddin R, et al. Troponin utilization in patients presenting with atrial fibrillation/flutter to the emergency department: Retrospective chart review. Int J Emerg Med 2011;4:25.
- O’Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: Executive summary: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: Developed in collaboration with the American College of Emergency Physicians and Society for Cardiovascular Angiography and Interventions. Catheter Cardiovasc Interv 2013;82:E1-27.
- Zimetbaum PJ, Josephson ME, McDonald MJ, et al. Incidence and predictors of myocardial infarction among patients with atrial fibrillation. J Am Coll Cardiol 2000;36:1223-1227.
- Androulakis A, Aznaouridis KA, Aggeli CJ, et al. Transient ST-segment depression during paroxysms of atrial fibrillation in otherwise normal individuals: Relation with underlying coronary artery disease.
J Am Coll Cardiol 2007;50:1909-1911. - Pradhan R, Chaudhary A, Donato AA. Predictive accuracy of ST depression during rapid atrial fibrillation on the presence of obstructive coronary artery disease. Am J Emerg Med 2012;30:1042-1047.
- Fengler BT, Brady WJ, Plautz CU. Atrial fibrillation in the Wolff-Parkinson-White syndrome: ECG recognition and treatment in the ED. Am J Emerg Med 2007;25:576-583.
- Conover MB. Diagnosis and management of arrhythmias associated with Wolff-Parkinson-White syndrome. Crit Care Nurse 1994;14:30-39; quiz 40-41.
- Uittenbogaart SB, et al. Burden of atrial high-rate episodes and risk of stroke: A systematic review. Europace 2017 Dec. 21. doi:1093/europace/eux356. [Epub ahead of print].
- Fuster V, Ryden LE, Cannom DS, et al. 2011 ACCF/AHA/HRS focused updates incorporated into the ACC/AHA/ESC 2006 Guidelines for the management of patients with atrial fibrillation: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines developed in partnership with the European Society of Cardiology and in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. J Am Coll Cardiol 2011;57:e101-e198.
- Zhang B, et al. Anterior-posterior versus anterior-lateral electrode position for external electrical cardioversion of atrial fibrillation: A meta-analysis of randomized controlled trials. Arch Cardiovasc Dis 2014;107:280-290.
- Kirchhof P, Eckardt L, Loh P, et al. Anterior-posterior versus anterior-lateral electrode positions for external cardioversion of atrial fibrillation: A randomised trial. Lancet 2002;360:1275-1279.
- Neumar RW, Otto CW, Link MS, et al. Part 8: Adult advanced cardiovascular life support: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2010;122(18 Suppl 3):S729-S767.
- Glover BM, Walsh SJ, McCann CJ, et al. Biphasic energy selection for transthoracic cardioversion of atrial fibrillation. The BEST AF Trial. Heart 2008;94:884-887.
- January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: Executive summary: A report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation 2014;130:2071-2104.
- Wyse DG, Waldo AL, DiMarco JP, et al. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med 2002;347:1825-1833.
- Van Gelder IC, Hagens VE, Bosker HA, et al. A comparison of rate control and rhythm control in patients with recurrent persistent atrial fibrillation. N Engl J Med 2002;347:1834-1840.
- Carlsson J, Miketic S, Windeler J, et al. Randomized trial of rate-control versus rhythm-control in persistent atrial fibrillation: The Strategies of Treatment of Atrial Fibrillation (STAF) study. J Am Coll Cardiol 2003;41:1690-1696.
- Fuster V, Ryden LE, Asinger RW, et al. ACC/AHA/ESC guidelines for the management of patients with atrial fibrillation: Executive summary. A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines and Policy Conferences (Committee to Develop Guidelines for the Management of Patients With Atrial Fibrillation): Developed in collaboration with the North American Society of Pacing and Electrophysiology. J Am Coll Cardiol 2001;38:1231-1266.
- Kotecha D, Holmes J, Krum H, et al. Efficacy of β blockers in patients with heart failure plus atrial fibrillation: An individual-patient data meta-analysis. Lancet 2014;384:2235-2243.
- Fromm C, Suau SJ, Cohen V, et al. Diltiazem vs. metoprolol in the management of atrial fibrillation or flutter with rapid ventricular rate in the emergency department. J Emerg Med 2015;49:175-182.
- Sagie A, Strasberg B, Kusnieck J, Sclarovsky S. Symptomatic bradycardia induced by the combination of oral diltiazem and beta blockers. Clin Cardiol 1991;14:314-316.
- Edoute Y, Nagachandran P, Svirski B, Ben-Ami H. Cardiovascular adverse drug reaction associated with combined beta-adrenergic and calcium entry-blocking agents. J Cardiovasc Pharmacol 2000;35:556-559.
- Stiell IG, Clement CM, Perry JJ, et al. Association of the Ottawa Aggressive Protocol with rapid discharge of emergency department patients with recent-onset atrial fibrillation or flutter. CJEM 2010;12:181-191.
- Scheuermeyer FX, Grafstein E, Stenstrom R, et al. Thirty-day and 1-year outcomes of emergency department patients with atrial fibrillation and no acute underlying medical cause. Ann Emerg Med 2012;60:755-765 e2.
- Cohn BG, Keim SM, Yealy DM. Is emergency department cardioversion of recent-onset atrial fibrillation safe and effective? J Emerg Med 2013;45:117-127.
- Vinson DR. The safety of cardioversion of recent-onset atrial fibrillation in emergency department patients. Ann Emerg Med 2012;60:134-135; author reply 135.
- Healey JS, Connolly SJ, Gold MR, et al. Subclinical atrial fibrillation and the risk of stroke. N Engl J Med 2012;366:120-129.
- Israel CW, Gronefeld G, Ehrlich JR, et al. Long-term risk of recurrent atrial fibrillation as documented by an implantable monitoring device: Implications for optimal patient care. J Am Coll Cardiol 2004;43:47-52.
- Michael JA, et al. Cardioversion of paroxysmal atrial fibrillation in the emergency department. Ann Emerg Med 1999;33:379-387.
- Stiell IG, Clement CM, Rowe BH, et al. Outcomes for emergency department patients with recent-onset atrial fibrillation and flutter treated in Canadian hospitals. Ann Emerg Med 2017;69:562-571 e2.
- Stiell IG, Clement CM, Symington C, et al. Emergency department use of intravenous procainamide for patients with acute atrial fibrillation or flutter. Acad Emerg Med 2007;14:1158-1164.
- Madrid AH, Moro C, Marin-Huerta E, et al. Comparison of flecainide and procainamide in cardioversion of atrial fibrillation. Eur Heart J 1993;14:1127-1131.
- Volgman AS, Carberry PA, Stambler B, et al. Conversion efficacy and safety of intravenous ibutilide compared with intravenous procainamide in patients with atrial flutter or fibrillation. J Am Coll Cardiol 1998;31:1414-1419.
- Holmes B, Heel RC. Flecainide. A preliminary review of its pharmacodynamic properties and therapeutic efficacy. Drugs 1985;29:1-33.
- Boriani G, Diemberger I, Biffi M, et al. Pharmacological cardioversion of atrial fibrillation: Current management and treatment options. Drugs 2004;64:2741-2762.
- Khan IA. Single oral loading dose of propafenone for pharmacological cardioversion of recent-onset atrial fibrillation. J Am Coll Cardiol 2001;37:542-547.
- Boriani G, Martignani C, Biffi M, et al. Oral loading with propafenone for conversion of recent-onset atrial fibrillation: A review on in-hospital treatment. Drugs 2002;62:415-423.
- Vinson DR, Lugovskaya N, Warton EM, et al. Ibutilide effectiveness and safety in the cardioversion of atrial fibrillation and flutter in the community emergency department. Ann Emerg Med 2018;71:96-108 e2.
- Kowey PR, Marinchak RA, Rials SJ, Filart RA, et al. Acute treatment of atrial fibrillation. Am J Cardiol 1998;81:16C-22C.
- Kowey PR, VanderLugt JT, Luderer JR. Safety and risk/benefit analysis of ibutilide for acute conversion of atrial fibrillation/flutter. Am J Cardiol 1996;78:46-52.
- Ellenbogen KA, Clemo HF, Stambler BS, et al. Efficacy of ibutilide for termination of atrial fibrillation and flutter. Am J Cardiol 1996;78:42-45.
- Boriani G, Biffi M, Capucci A, et al. Conversion of recent-onset atrial fibrillation to sinus rhythm: Effects of different drug protocols. Pacing Clin Electrophysiol 1998;21(11 Pt 2):2470-2474.
- Capucci A, Lenzi T, Boriani G, et al. Effectiveness of loading oral flecainide for converting recent-onset atrial fibrillation to sinus rhythm in patients without organic heart disease or with only systemic hypertension. Am J Cardiol 1992;70:69-72.
- Galve E, Rius T, Ballester R, et al. Intravenous amiodarone in treatment of recent-onset atrial fibrillation: Results of a randomized, controlled study. J Am Coll Cardiol 1996;27:1079-1082.
- Lafuente-Lafuente C, Valembois L, Bergmann JF, et al. Antiarrhythmics for maintaining sinus rhythm after cardioversion of atrial fibrillation. Cochrane Database Syst Rev 2015(3):CD005049.
- Falk RH, Knowlton AA, Bernard SA, et al. Digoxin for converting recent-onset atrial fibrillation to sinus rhythm. A randomized, double-blinded trial. Ann Intern Med 1987;106:503-506.
- Jordaens L, Trouerbach J, Calle P, et al. Conversion of atrial fibrillation to sinus rhythm and rate control by digoxin in comparison to placebo. Eur Heart J 1997;18:643-648.
- [No authors listed.] Intravenous digoxin in acute atrial fibrillation. Results of a randomized, placebo-controlled multicentre trial in 239 patients. The Digitalis in Acute Atrial Fibrillation (DAAF) Trial Group. Eur Heart J 1997;18:649-654.
- Ganga HV, Noyes A, White CM, Kluger J. Magnesium adjunctive therapy in atrial arrhythmias. Pacing Clin Electrophysiol 2013;36:1308-1318.
- Rasmussen HS, Thomsen PE. The electrophysiological effects of intravenous magnesium on human sinus node, atrioventricular node, atrium, and ventricle. Clin Cardiol 1989;12:85-90.
- Shah SA, et al. Impact of magnesium sulfate on serum magnesium concentrations and intracellular electrolyte concentrations among patients undergoing radio frequency catheter ablation. Conn Med 2008;72:261-265.
- Tercius AJ, Kluger J, Coleman CI, White, CM. Intravenous magnesium sulfate enhances the ability of intravenous ibutilide to successfully convert atrial fibrillation or flutter. Pacing Clin Electrophysiol 2007;30:1331-1335.
- Kalus JS, Spencer AP, Tsikouris JP, et al. Impact of prophylactic i.v. magnesium on the efficacy of ibutilide for conversion of atrial fibrillation or flutter. Am J Health Syst Pharm 2003;60:2308-2312.
- Patsilinakos S, Christou A, Kafkas N, et al. Effect of high doses of magnesium on converting ibutilide to a safe and more effective agent. Am J Cardiol 2010;106:673-676.
- Barrett TW, Martin AR, Jenkins CA, et al. A clinical prediction model to estimate risk for 30-day adverse events in emergency department patients with symptomatic atrial fibrillation. Ann Emerg Med 2011;57:1-12.
- Barrett TW, Jenkins CA, Self WH. Validation of the Risk Estimator Decision Aid for Atrial Fibrillation (RED-AF) for predicting 30-day adverse events in emergency department patients with atrial fibrillation. Ann Emerg Med 2015;65:13-21.e3.
- Gage BF, van Walraven C, Pearce L, et al. Selecting patients with atrial fibrillation for anticoagulation: Stroke risk stratification in patients taking aspirin. Circulation 2004;110:2287-2292.
- Olesen JB, Torp-Pedersen C, Hansen ML, Lip GY. The value of the CHA2DS2-VASc score for refining stroke risk stratification in patients with atrial fibrillation with a CHADS2 score 0-1: A nationwide cohort study. Thromb Haemost 2012;107:1172-1179.
- Lip GY, Nieuwlaat R, Pisters R, et al. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: The euro heart survey on atrial fibrillation. Chest 2010;137:263-272.
- Habert JS. Minimizing bleeding risk in patients receiving direct oral anticoagulants for stroke prevention. Int J Gen Med 2016;9:337-347.
This article provides an overview of atrial fibrillation (AF) and evidence-based guidance on controversial aspects of AF workup and management in the emergency department. The evidence is provided to help safely reduce unnecessary testing and expand the emergency provider’s management armamentarium to include electrical and pharmacologic conversion in recent-onset AF patients.
Subscribe Now for Access
You have reached your article limit for the month. We hope you found our articles both enjoyable and insightful. For information on new subscriptions, product trials, alternative billing arrangements or group and site discounts please call 800-688-2421. We look forward to having you as a long-term member of the Relias Media community.



