Pediatric Airway Management
October 1, 2017
Related Articles
-
Infectious Disease Updates
-
Noninferiority of Seven vs. 14 Days of Antibiotic Therapy for Bloodstream Infections
-
Parvovirus and Increasing Danger in Pregnancy and Sickle Cell Disease
-
Oseltamivir for Adults Hospitalized with Influenza: Earlier Is Better
-
Usefulness of Pyuria to Diagnose UTI in Children
AUTHORS
Julie Furmick, DO, Pediatric Resident, University of Arizona, Tucson
Louise Malburg, MD, Pediatric Resident, University of Arizona, Tucson
Aaron Leetch, MD, FACEP, Assistant Professor of Emergency Medicine and Pediatrics, Residency Director, Combined Emergency Medicine and Pediatrics Residency, University of Arizona, Tucson
PEER REVIEWER
Charles Nozicka, DO, FAAP, FACEP, FAAEM, Division Director, Pediatric Emergency Medicine, Advocate Children's Hospital, Park Ridge, IL; Clinical Professor of Emergency Medicine, Rosalind Franklin University, Libertyville, IL
EXECUTIVE SUMMARY
- Children have large heads relative to their body size with prominent occiputs, leading to hyperflexion of the neck, and subsequent airway obstruction when positioned on a flat surface. Children also have larger tongues and smaller mandibles, with shorter, narrower tracheas and larynges, respectively, and a funnel-shaped airway that narrows inferiorly to the cricoid.
- The cricoid ring is elevated at birth and is located at the level of the C4 vertebra. As the child grows, the airway elongates and the cricoid ring moves inferiorly, making its way to C6 by adulthood. The cricothyroid space emerges with this migration, creating an accessible membrane for potential cricothyrotomy only after the age of 10 years.
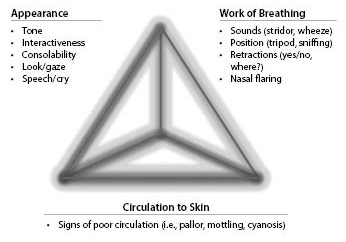
- The Pediatric Assessment Triangle addresses appearance, work of breathing, and circulation. Each component is broken down further and evaluated separately, looking for specific characteristics.
- Anaphylaxis is an acute, hypersensitivity reaction that can cause rapid onset of systemic symptoms after exposure and involve two or more organ systems. In the majority of cases, there is cutaneous involvement with flushing, pruritus, urticaria, and/or angioedema.
Pediatric patients frequently present with respiratory complaints. Fortunately, most children respond well to simple medical interventions. Understanding a child's anatomic and physiologic differences is critical to effectively preventing respiratory failure and stabilizing a child when it occurs.
— Ann M. Dietrich, MD, FAAP, FACEP
Respiratory failure is the most common cause of cardiorespiratory arrest in pediatric patients. In 2010, respiratory disorders were the second most common cause of pediatric emergency department visits1 and, in general, respiratory compromise is the most common cause for non-surgical pediatric ICU admissions.2,3,4 Often, emergent airway control is vital to successful pediatric resuscitation in the emergency department. However, compared to adults, children have significant anatomical and physiological differences that can make airway management difficult.5 This review focuses on management of the pediatric airway before, during, and after resuscitation, as well as difficult airway management.
Anatomy and Physiology
Pediatric Anatomy
Pediatric airway anatomy differs from adult anatomy and directly affects airway management. Children have large heads relative to their body size with prominent occiputs, leading to hyperflexion of the neck, and subsequent airway obstruction when positioned on a flat surface. In addition, children have larger tongues and smaller mandibles, with shorter, narrower tracheas and larynges, respectively. In contrast to adults in whom the narrowest region of the airway is the glottis with a cylindrical shape, children have a funnel-shaped airway, narrowing inferiorly to the cricoid.6 This becomes clinically relevant in the case of croup, where the anatomical swelling occurs at the level of the cricoid ring.7 Prominent adenoid and tonsillar tissue also can be problematic, particularly in preschool-aged children, secondary to this anatomical default.8 All of these factors contribute to loss of upper airway space, difficulty ventilating with a bag valve mask (BVM), and poor visibility during intubation.
The level, position, shape, and stiffness of the epiglottis and larynx differ in children compared to adults. The cricoid ring is elevated at birth and is located at the level of the C4 vertebra, but as the child grows, the airway elongates and the cricoid ring moves inferiorly, making its way to C6 by adulthood. The cricothyroid space emerges with this migration, creating an accessible membrane for potential cricothyrotomy only after the age of 10 years.7 The vocal cords are found just superior to the cricoid ring at C3-C4 and are angled in a posterior-superior direction rather than a 90° angle to the epiglottis as seen in adults. The epiglottis is floppy, loose, and U-shaped in children compared to the rigid and flat appearance in adults; this makes it more difficult to manipulate the epiglottis out of view during intubation by its attached ligamentous structures.
Airway Physiology
The pediatric airway also differs physiologically from that of adults. Children consume more oxygen at rest and have lower functional residual capacities, leading to a more rapid onset of hypoxemia and hypercapnia when apneic.9 Thus, preoxygenation prior to intubation does not sustain oxygen saturations in children as much as it does in adults. Also, there is an increased airway resistance to flow driven by Poiseuille’s law, in which the resistance to flow is inversely proportional to the length of the airway and the radius raised to the fourth power. (See Figure 1.) As a result, a small change in the diameter of the child’s airway, independent of the cause (mucus from respiratory syncytial virus, structural abnormalities, post-intubation edema, etc.), can have devastating consequences on air movement.6
Figure 1.Poiseuille’s Law |
|||
|
Healthy Airway |
Constriction of Airway |
Resistance |
|
|
Child Radius = 4 |
 |
 |
16 x |
|
Adult Radius = 8 |
 |
 |
3 x |
|
Resistance to flow is inversely related to the radius raised to the fourth power. Source: Author created. |
|||
The resting metabolic demand for an infant is two to three times higher than for an adult. Although mature alveoli are present at 36 weeks’ gestation, a great deal of alveolar septation and microvascular maturation occurs after birth and continues until full development at 5 years of age. Therefore, the surface area for gas exchange is decreased in pediatric patients.10,11
Recognition of Respiratory Difficulty
Many issues can lead to respiratory failure in a child, and the emergency provider must recognize a child in respiratory distress before the condition progresses to respiratory failure, shock, and, ultimately, cardiopulmonary arrest.
Respiratory distress often can be difficult to recognize, especially in a young, nonverbal child; for example, agitation may be the first sign of hypoxemia.12 Independent of the cause, infants and children often become tachypneic and tachycardic, with compensatory signs of respiratory distress including subcostal and intercostal retractions, head bobbing, nasal flaring, and grunting.10,13 Various respiratory sounds can help distinguish the etiology of respiratory distress. When present, stridor often indicates an upper airway obstruction, usually above or near the level of the glottis. Based on onset, tone, and phase, one can differentiate between the anatomic, obstructive, and infectious causes of stridor. Foreign body, anaphylaxis, laryngomalacia, laryngotracheitis/croup, bacterial tracheitis, and epiglottitis all are common causes of stridor and will be discussed in more detail later. Wheezing in respiratory distress often indicates lower airway (subglottic) pathology, such as asthma or bronchiolitis. None of these symptoms will come in isolation and all need to be taken within the context of the patient and his or her clinical presentation.
If unrecognized or undermanaged, respiratory distress may lead to respiratory failure. It also is important to note that although primary lung pathologies do account for a large percentage of patients presenting with respiratory disease, respiratory failure can ensue secondary to heart failure as well. Once a child has progressed to respiratory failure, tachypnea and tachycardia give way to shallow breathing, bradycardia, and altered mental status. In an infant, altered mental status may present as listlessness. A baby between 9 months and 18 months of age should have some degree of stranger anxiety, especially if the child is sick and fussy. A child who is listless and is not fighting examination, straight cath, or intravenous (IV) attempts is an ominous sign.14 Further decline in clinical status includes hypercapnia, cyanosis, and acidosis secondary to poor oxygen delivery to the tissues; this will lead to shock and, ultimately, cardiopulmonary arrest if not corrected.15
Initial Exam and Airway Assessment
When a child presents in respiratory distress, the emergency assessment should occur in an organized and stepwise fashion. The general “observational” assessment should occur quickly, giving the provider an idea of the patient’s vital signs, level of consciousness, and overall work of breathing. This is followed by the primary assessment, which includes the ABCs (airway, breathing, and circulation), then the secondary survey, and finally the tertiary assessment. The goal of obtaining a general impression of the patient prior to the initial assessment is to determine if the child is “sick” or “not sick.” Does this patient need immediate medical intervention?
The Pediatric Assessment Triangle (PAT) is a simple tool based on observational signs only that can be used to identify high-acuity patients quickly at the first point of contact. Originally, it was developed as a tool to standardize the initial assessment of children for all levels of providers,16 and it since has become the cornerstone in the assessment of a sick child. In 2013, Horeczko et al conducted a study in which 528 children were assessed and likelihood ratios were found for instability; those children found to be stable by initial PAT scores were 10 times more likely to be stable on follow-up assessment.17,18
The PAT addresses appearance, work of breathing, and circulation. Then each component is broken down further and evaluated separately, looking for specific characteristics. (See Figure 2.) The findings in each arm of the PAT can be combined to categorize the patient into further levels of physiologic despair: respiratory distress, failure, shock, central nervous system failure/metabolic derangement, or cardiopulmonary failure. Abnormality noted in any arm of the triangle indicates an unstable child who requires immediate clinical intervention. If the patient does not require immediate intervention, then a more thorough primary assessment may be performed. Similar to the PAT, this is a systematic approach to evaluating life-threatening abnormalities and typically has been taught via the ABC mnemonic for airway, breathing, and circulation. A simple way to assess airway patency in patients is to have them speak or assess the quality of their cry. This will help assess both airway and neurologic status. If the patient has a muffled or “hot potato” voice, this is suggestive of an upper airway obstruction. Other evidence of upper airway obstruction includes drooling, stridor, and dyspnea. Breathing and quality of breath often are assessed similarly in the PAT and the primary assessment, and distress noted by grunting, nasal flaring, and retractions. Respiratory distress accompanied by wheezing, typically described as a diffuse, high-pitched whistling sound, usually indicates a lower airway obstruction. This usually is caused by pathology such as bronchiolitis, asthma, or pneumonia, but also can include foreign body obstruction or mass. Finally, the provider needs to assess circulation quickly. This can be done by evaluating pulses in all four extremities, evaluating central capillary refill (forehead or sternum),19 and looking at the skin temperature and color. It is important to check pulses in all four extremities, especially in young infants, to rule out coarctation of the aorta, discussed in more detail under differential diagnosis.
Figure 2.The Pediatric Assessment Triangle |
|
Adapted from: Horeczko T, Enriquez B, McGrath NE, et al. The Pediatric Assessment Triangle: Accuracy of its application by nurses in the triage of children. J Emerg Nurs 2013;39:182-189. |
After the patient’s ABCs have been assessed and stabilized, the provider should complete the secondary survey, which includes a complete history and physical exam. The history can be completed most efficiently using the mnemonic SAMPLE:
- Signs and symptoms,
- Allergies,
- Medications,
- Past medical history (include relevant history such as prematurity, known anatomic anomalies, and chronic respiratory, cardiac, or neuromuscular diseases),
- Last meal (includes all oral intake, not just food),
- Events leading up to current illness.
Labs tests, imaging, and other diagnostic entities usually occur at the same time as the above interventions, but are considered part of the tertiary assessment. In the evaluation of a patient in respiratory distress, an important lab to start is a venous blood gas to assess both oxygenation and ventilation status. If a bacterial infection is suspected, blood, urine, and sputum cultures may be indicated to help identify the source. A portable chest X-ray can be a high-yield imaging method and can be obtained simultaneously with resuscitation. If cardiac abnormalities are suspected, consider electrocardiogram and/or echocardiogram.
Differential Diagnosis
The differential diagnosis for a child in respiratory distress is very broad. Table 1 outlines common causes of pediatric respiratory distress based on time frame. For the purposes of discussion, this paper will approach the various etiologies grouped by the onset of symptoms.
Table 1.Differential Diagnosis of Respiratory Distress Based on Timing of Symptoms |
|
|
Onset of Symptoms |
Differential |
|
Minutes |
Anaphylaxis, foreign body aspiration |
|
Hours |
Viral infection, bacterial infection |
|
Days |
Bacterial infection, viral infection |
|
Weeks |
Enlarging mass, congenital structural defects |
|
Months |
Subglottic stenosis, congenital structural defects |
|
SOURCE: Author created. |
|
Anaphylaxis is an acute, hypersensitivity reaction that can cause rapid onset of systemic symptoms with potential for airway compromise. The most common triggers include food (milk, soy, peanut, fish, wheat, egg), medications (penicillin, nonsteroidal anti-inflammatory drugs), vaccines, latex, and insect venom. Symptoms begin within minutes to hours of exposure and must involve two or more organ systems. In the majority of cases, there is cutaneous involvement with flushing, pruritus, urticaria, and/or angioedema.
The second most common system involved is the respiratory tract with both upper and lower airway symptoms, such as throat itchiness or tightness, dysphonia, stridor, shortness of breath, chest tightness, and/or wheezing. There are several different ways to describe wheezing, but important attributes that often help to distinguish the pathology are the pitch, timing, and location. This often is labeled as polyphonic compared to monophonic wheezing. In monophonic wheezing, everything — pitch, location, and timing — is constant. This indicates fixed obstruction in medium-sized airways, and symptoms are unchanged until the obstruction is removed. Polyphonic wheezing is dynamic and represents an airway of someone with asthma or bronchiolitis. It is described as loud, diffuse, and multifaceted. Pitch may vary depending on bronchoconstriction or mucus plugging present in various locations.20
Polyphonic wheezing may be difficult to identify in infants or young children, and may present as a sudden change in behavior or appearing anxious or frightened. Gastrointestinal symptoms also can be prominent, with nausea, abdominal pain, vomiting, or diarrhea. Cardiovascular involvement occurs in about 45% of cases with tachycardia, chest pain, hypotension, and shock. Once identified, management of anaphylaxis involves immediate removal of the suspected allergen, if possible, and prompt administration of epinephrine (0.01 mg/kg of 1:1000 dilution; max dose 0.5 mg for an adult or 0.3 mg for a child) via intramuscular injection to the lateral thigh. This dose may be repeated every five to 15 minutes as needed. Other treatment options include H1 antihistamines or glucocorticoids, but these are second-line therapies that should not delay the administration of epinephrine. Additionally, the patient should have continuous cardiopulmonary monitoring and should receive supplemental oxygen and IV fluids. Upon resolution of symptoms, the patient should be observed for at least four to six hours for development of a biphasic reaction.21,22,23
Foreign body aspiration is potentially life threatening and is very common in children; according to the National Safety Council statistics, in 2011, foreign body aspiration and mechanical suffocation accounted for 3.3% of all deaths in children in the United States younger than 4 years of age.2,24 Clinical presentation can vary; the most common presentation is coughing, gagging, shortness of breath with stridor, or unilateral, monophonic wheezing on exam. In 90% of patients presenting with foreign body aspiration, one or more of the above symptoms is present.25,26 However, it is important to remember that history can vary and approximately 50% of cases occur without a witnessed choking event.
Physical exam depends on location and degree of obstruction but can be completely normal.24 Chest X-ray findings are nonspecific but can include air trapping, atelectasis, and consolidations. In fact, in a study in which 1,500 patients were examined, including 584 with foreign body aspiration, Yang et al found that chest X-ray only demonstrated a sensitivity and specificity of 68% and 67%, respectively, while computed tomography (CT) was found to have a sensitivity and specificity of 99.8% and 99.8%, respectively.27 The most commonly aspirated foreign bodies in the United States are organic food items, such as peanuts,26 which are radiolucent, demonstrating why X-ray might not be the best study of choice immediately after the aspiration event. Still, there clearly is an increase in radiation exposure with CT, and there often can be a clinical concern in laying a child flat who has potential airway compromise. For this reason, the clinical picture often can be enough to prompt a diagnostic laryngoscopy/ bronchoscopy by a surgeon.
Treatment depends on the degree of obstruction. Partial obstruction is indicated by a patient who can cough, cry, or speak in some capacity. With the exception of infants, the patient automatically will hold himself or herself in a position that allows for maximum air exchange, and retrieval of the foreign body should occur only in a controlled setting under anesthesia if resources allow. Complete obstruction is a medical emergency, as this blocks all air exchange, and results in the complete loss of phonation with absent breath sounds on auscultation.28 Figure 3 delineates the treatment appropriate for foreign body aspiration.
Figure 3.Suggested Treatment Algorithm for Foreign Body Aspiration |
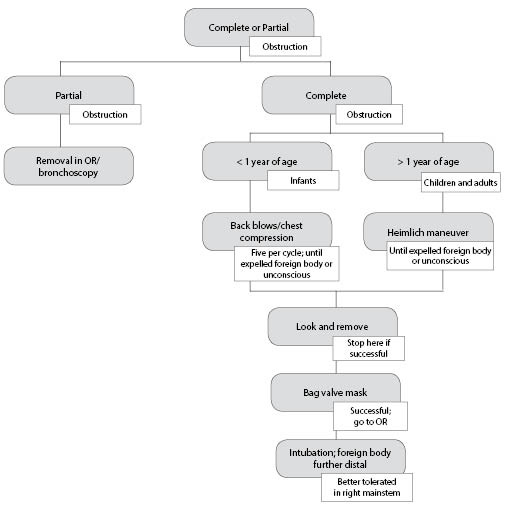 |
|
SOURCE: Author created. |
Bacterial tracheitis is caused by a bacterial infection of the supraglottic region (often a complication of a preceding viral infection involving the trachea) leading to a febrile, toxic-appearing child with severe biphasic stridor and copious amounts of purulent sputum. This often affects children between 3 months and 6 years of age, and common pathogens include Staphylococcus aureus, Streptococcus pneumoniae, and Haemophilus influenzae type b (unvaccinated patient). This diagnosis carries a high intubation rate (51-93%),25,26 and a high percentage of these patients have concurrent lower airway disease (more than 50% have pneumonia or tracheobronchitis). Once the airway is stabilized and blood and sputum cultures are obtained, these patients should be started on parenteral antibiotics with broad-spectrum coverage.29,30
Epiglottitis is another very serious and potentially life-threatening cause of stridor. In this disease process, the epiglottis and surrounding supraglottic structures become edematous, leading to possible occlusion of the airway and decreased clearance of oral secretions. Historically, the main pathogen responsible for this disease process was H. influenzae type b; however, since the introduction of the Hib vaccine, the incidence of epiglottitis has decreased dramatically. The more recent pathogens implicated in epiglottitis include non-typeable Haemophilus parainfluenzae, S. pneumoniae, S. aureus, or Klebsiella. Epiglottitis can affect all ages, but is seen most commonly in those between 2 and 6 years of age; the patient will be ill-appearing and febrile, with a history of progressive onset respiratory distress and stridor. This history can help distinguish epiglottitis from tracheitis, as epiglottitis may manifest as a primary infection, and tracheitis often is preceded by a viral infection.30
Additionally, the patient with epiglottitis will present with significant drooling and a muffled voice due to supraglottic edema, and the patient with tracheitis will have a cough. On physical exam, the patient often leans forward with his or her jaw protruding (tripod position) in an effort to augment breathing and avoid swallowing secretions. The classic finding of a “thumbprint sign” may be seen on a lateral radiograph of the neck, although this is not necessary for diagnosis. Diagnosis is made with direct visualization with laryngoscopy of an erythematous and edematous epiglottis, but the procedure should be performed only in a controlled setting, such as in an operating room, because of the significant risk of laryngospasm. In fact, direct visualization of the epiglottis using a tongue blade is highly discouraged, as this could also lead to life-threatening airway occlusion. Additionally, any anxiety-provoking procedures, such as IV placement, blood draws, or X-ray, should be avoided until the patient’s airway has been secured.31,32 Expert opinion and traditional teaching dictates that such patients should be left in a position of comfort and intubated only when they show signs of respiratory failure.
Laryngotracheitis or “croup” is caused by an infectious inflammation of the glottis and subglottic structures leading to a barking cough and stridor. The most common pathogen leading to these symptoms is parainfluenza type 1, but others include parainfluenza type 2, respiratory syncytial virus, influenza, and herpes simplex type 1. The presentation for croup is very consistent; the infant typically is between 6 months and 1.5 years, with mild respiratory distress and a barking cough. In fact, the cough is so specific that when absent, the provider should consider an alternative diagnosis. These patients often have low-grade fevers, rhinorrhea, and mild fussiness. They generally are not ill-appearing and often are stridorous only when upset.
Since croup is such a benign and rapidly reversible condition, most patients’ symptoms improve by the time they reach the emergency department after exposure to the cold night air and being placed in an upright position.30 However, if a patient with croup presents with significant stridor at rest, this is a concerning sign and treatment should be initiated as soon as possible. A single dose of dexamethasone, either oral or intramuscular, has been shown to improve symptoms within six hours.31 Racemic epinephrine with or without oxygen supplementation may be used for symptomatic care while awaiting the effect of the corticosteroid. Some studies have suggested a transient benefit with use of heliox in patients with moderate to severe croup; however, there is not adequate evidence to suggest it is superior to oxygen supplementation.33 If standard management with corticosteroids and racemic epinephrine fails to improve symptoms, other diagnoses such as bacterial tracheitis should be considered.29
A less common but important cause of stridor to consider is a laryngeal obstruction from an enlarging mass, such as a hemangioma or a papilloma. In the case of recurrent respiratory papillomatosis, infection by human papilloma virus strain 6 or 9 leads to papilloma development within the respiratory tract. Papillomas can develop anywhere within the respiratory tract and affect patients of any age, but are most troublesome in the larynx of small infants in whom the diameter of the airway is very limited. The papillomas may grow fast and lead to difficulty breathing, coughing, and swallowing and to stridor. Transmission is thought to be through the vaginal canal of affected mothers during childbirth.34 Papillomas and other masses represent mechanical obstructions that generally do not improve with medications. Instead, the patient should be placed in a position of comfort and preparation should be made for surgical management.
Several congenital syndromes affect airway anatomy. Laryngomalacia is secondary to a congenital abnormality of the supraglottic structures, leading to collapse of these structures during inspiration. The infant is young at presentation (2-4 weeks) and overall is well appearing. In this presentation, the stridor is often positional: worsened in the supine position and improved when prone. This instability can be worsened by a simple viral illness or other respiratory infection. Depending on the severity, intervention can range from observation to surgery. Additional syndromes that can lead to difficult intubation are listed in Table 2.35
Table 2.Congenital Syndromes and Their Anatomical Characteristics That Contribute to a Difficult Intubation With Suggested Interventions |
||
|
Syndrome |
Anatomical Characteristics |
Interventions |
|
Trisomy 21 |
|
|
|
Pierre Robin sequence |
|
|
|
Treacher Collins syndrome |
|
|
|
Beckwith-Wiedemann syndrome |
|
|
|
Klippel-Feil syndrome |
|
|
|
SOURCE: Author created. |
||
Initial Therapy
Management of a child in respiratory distress should occur in a stepwise fashion. In some cases, early airway interventions may stave off or even prevent intubation. Patients with signs of mild respiratory distress limited to tachypnea and slight retractions may benefit from simple support methods, such as proper airway positioning and nasal suctioning. Pulse oximetry generally is a reliable measurement of oxygen saturation and can guide management. When available, forehead probes are more reliable in the setting of hypotension, hypothermia, or under perfusion.36,37 The airway management technique of choice depends on the severity of presentation. The stepwise management of the pediatric airway discussed below may be hurried, depending on the severity of the child’s distress.
Nasal cannula is a low-flow oxygen system that delivers slightly higher than room air fraction of inspired oxygen (FiO2), with flow ranges from 2-6 L/minute. Higher than 6 L/minute has demonstrated minimal increases in inspired oxygen. If no improvement is seen, the simple face mask can be used. This is a non-form-fitting plastic mask with no bag reservoir and offers flows slightly higher, 4-10 L/minute; FiO2 varies but has been estimated around 50%. A non-rebreather apparatus is similar, but has a 500 to 1,000 mL bag reservoir from which the patient can inhale during inspiration. FiO2 has been estimated to be as high as 70% at flow between 10-15 L/minute on this device.38
For patients who continue to worsen, noninvasive positive pressure ventilation, such as high-flow nasal cannula (HFNC) and continuous positive airway pressure (CPAP), can provide respiratory support.
HFNC delivers heated, humidified, blended air delivering flow > 2 L/minute. This device is capable of delivering high concentrations of oxygen, washing out the nasopharyngeal dead space leading to increased oxygen within the alveoli, and providing continuous distending airway pressures. Some small, prospective studies have identified positive clinical effects of HFNC on pulse oximetry, PaO2, and length of stay, but nothing has been able to demonstrate clinical significance. However, trends seem to be particularly evident in those with bronchiolitis. In one small, randomized, controlled trial, children with severe pneumonia received either HFNC or CPAP. No significant difference was observed between the treatments.39 Several other smaller retrospective studies demonstrated similar findings. When a patient presents to the emergency department in respiratory distress with impending respiratory failure, it is most important to know who is likely to fail HFNC and require intubation.40 These factors are illustrated in Table 3.41
Table 3.Children at Risk for Failing High-flow Nasal Cannula, Requiring Intubation |
|
Triage respiratory rate > 90% for age |
|
Initial venous PCO2 > 50 mmHg |
|
Initial venous pH < 7.30 |
|
Adapted from: Kelly GS, Simon HK, Sturm JJ. High-flow nasal cannula use in children with respiratory distress in the emergency department. Pediatr Emerg Care 2013;29:888-892. |
When a patient’s clinical status decompensates to respiratory failure, BVM ventilation is the first and most basic step in airway management.42 It is important to ensure that the BVM has a one-way exhalation port so all the tidal volume is drawn from the oxygen reservoir without mixing with room air. Oral and nasopharyngeal airways can be used if the child demonstrates signs of upper airway obstruction from a large tongue or adenoidal tissue.
Decision to Intubate
Indications for intubation include: 1) failure of airway maintenance and protection; 2) failure of ventilation and oxygenation; and 3) anticipated clinical course requiring intubation.12
Failure to protect the airway can be assessed quickly. An alert patient who is crying or speaking in an unobstructed voice is strong evidence of a protected, patent airway. The presence of a gag reflex has not been demonstrated to ensure airway protection; however, the lack of a gag reflex may indicate the need for intubation. Pooling of secretions in the patient’s posterior pharynx is more indicative of poor airway protection and requirement of intubation.7 During in-hospital pediatric code arrests, tracheal intubation during cardiopulmonary resuscitation has been associated with decreased survival to hospital discharge as well as return of spontaneous circulation (ROSC).43 The reason is unknown and likely is multifactorial. It has been suggested that this is secondary to interruptions in chest compressions and other vital interventions, like medication administration and defibrillation. Still, the decision to intubate is best made clinically with appropriate consideration of preparation, technique, and anticipated clinical course.
Rapid Sequence Intubation
Most intubations in the emergency department occur as rapid sequence intubations (RSI). The purpose of this procedure is to cause an unconsciousness state quickly and, often (but not always), to cause paralysis to allow for endotracheal intubation without the use of positive pressure ventilation (PPV). The avoidance of PPV is particularly important in the emergency setting when the time of the last meal often is unknown and the risk of aspiration is high.44
The steps of RSI can be summarized by the seven Ps:
- Preparation;
- Preoxygenation;
- Pretreatment;
- Paralysis with induction;
- Protection and positioning;
- Placement with proof;
- Post-intubation care.
Preparation
The initial step in intubation is to obtain a history (SAMPLE mnemonic), paying attention to any congenital syndromes with known anatomical variants that may be challenging during endotracheal intubation.45,46 (See Table 2.) Next, examine the patient for evidence of a potentially difficult airway and prepare all necessary equipment.
The Mallampati score often is used to predict difficult airways by classifying the amount of space in the patient’s mouth based on the degree of visibility of oropharyngeal structures. This test was designed and studied primarily for adults and may be difficult to perform in the pediatric patient in respiratory distress, as it requires patient participation. In children where the Mallampati score could be obtained, prediction of difficult laryngoscopy seemed to be reliable.47 The evaluation is made while the patient is in a sitting position with the head in a neutral position, mouth fully open and tongue extended, without phonation. Classes 3 and 4 are predictors of a difficult airway. Class 3 reveals the soft palate and base of the uvula, while the soft palate is not visualized in Class 4. Studies have shown poor sensitivity and specificity with this test alone, but improvement is seen when used in conjunction with other bedside tests.7,48
The LEMON mnemonic is useful to help the emergency provider identify multiple risk factors pertaining to a difficult airway in an emergent situation. L stands for look, meaning to simply look at the patient externally. A provider’s general gestalt of difficult intubation is fairly specific. E = 3-3-2 is a measurement based on the thyromental distance. The first 3 assesses for mouth opening. A normal patient can open his/her mouth wide enough to accommodate three of his or her own fingers between the upper and lower central incisors. The second 3 evaluates the length of the mandibular space; three of the patient’s fingers should fit under his or her chin. The 2 evaluates the position from the glottis to the base of the tongue; two fingers should fit between the neck-chin junction and the hyoid bone.49 Finally, the Cormack-Lehane grading system is based on four grades of direct laryngoscopic views and may predict the difficulty of passing an endotracheal tube (ETT). A score of 3 or 4 is associated with a difficult intubation with visualization of only the epiglottis or only the soft palate, respectively. However, this is a less useful preparatory step for emergency providers, as they often are committed to intubation by this step.
After physical assessment of the airway, the next step is to select appropriate equipment. A mnemonic to remember the necessary equipment is SOAPME:
- Suction device,
- Oxygen,
- Airway equipment,
- Pharmacologic agents,
- Monitoring Equipment.
To determine the ETT size, one may use the length-based Broselow-Luten equipment chart or the formula (16 + age in years)/4 for uncuffed tubes. The depth of tube insertion also is listed on the Broselow-Luten chart or may be estimated by multiplying the ETT size by 3. In premature infants, the size may be estimated by dividing the gestational age in weeks by 10. Uncuffed ETTs may be used in the neonatal period because of the anatomical and functional seal provided by the subglottic area; otherwise, cuffed ETTs are the preferred choice. Historically, cuffed tubes were avoided, but because of the risk of subglottic stenosis as result of high cuff pressures, the modern ETT has decreased cuff pressure and increased cuff size, thus increasing its safety.7,46,50,51 When using a cuffed tube, the calculated ETT size should be decreased by 0.5 to allow for the size of the balloon.
An advanced tool is the fiberoptic stylet, which provides an advantage in the airway with a significantly obstructed view.12,52 Laryngeal mask airways also can serve as rescue devices in the case of difficult intubations and can be prepared at the bedside.
The two most commonly used laryngoscope blades are the Macintosh (curved) and the Miller (straight). The Macintosh blade is inserted in the vallecula to indirectly lift the epiglottis out of view. This technique generally is not as effective in patients younger than 2 years of age because of the increased size and floppiness of the epiglottis. In this case, the Miller blade can provide better visualization, as it directly lifts the epiglottis from view.46 (See Figure 4.) Miller laryngoscope was found to have advantages over the other laryngoscopes in regard to glottic view, duration of successful intubation, number of attempts, dental trauma severity, need for additional maneuvers, and ease of use.53
Figure 4.View With Miller and Macintosh Blades |
|
|
Miller Blade |
Macintosh Blade |
 |
 |
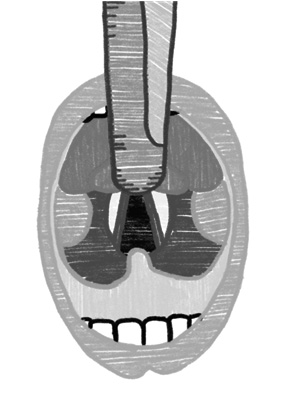 |
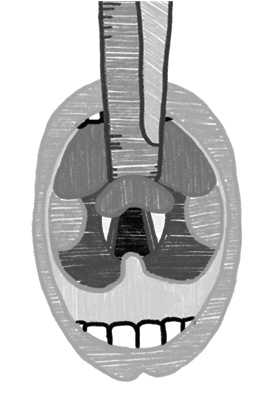 |
|
SOURCE: Author created. |
|
In addition to the blade, one must decide which type of laryngoscope (direct vs. video) to use and ensure it is functioning properly. In a surveillance study of pediatric intubation practices in the emergency department over a 10-year period (2002-2012), there was a clear increase in use of video laryngoscopy over direct laryngoscopy, with use of the video method in up to 50% of children in the last year of the study. The advantages of video laryngoscopy include better glottic views, the ability for colleagues to watch the attempt (which may decrease incidence of esophageal intubation), and for some devices it can be converted easily to direct visualization in the event of video failure. Video laryngoscopy also has been associated with improved first-pass success, which has direct effects on patient outcome.54
However one series, in manikins, has shown the standard straight or curved laryngoscope blades including the CMAC were associated with shorter procedural time and higher success rate when compared with indirect videolaryngoscopy with an unconventional blade design such as the GlideScope in both experienced and inexperienced users.55
Although the primary person in charge of the intubation is selecting airway equipment and ensuring its proper functioning, other team members may assist in placing the patient on continuous cardiorespiratory monitors with automated blood pressure monitoring, obtaining reliable intravenous access, and drawing up and labeling any anticipated pharmacologic agents.
Preoxygenation
The purpose of preoxygenation is to denitrogenate the lungs and maximize partial pressure of arterial oxygen to provide a buffer during the time it takes for intubation. Preoxygenation extends the time it takes for the patient’s oxygen saturation to decrease from 100% to 90%, which is referred to as “safe apnea time.” To fully maximize preoxygenation, the patient should receive 100% oxygen for three minutes (may be obtained within one minute in infants and toddlers) when possible. A common method of administration in the emergency department is via a non-rebreather mask. This should be placed at its maximal flow rate to improve the FiO2 to > 90% vs. 70% at the standard 15 L/minute. In the case of a critically ill patient with difficulty achieving adequate preoxygenation, nasopharyngeal airways, oral airways, or noninvasive PPV can be used. If this fails, a BVM device may be used.12,40 Following adequate preoxygenation, a healthy 10 kg child will take less than four minutes to desaturate to less than 90%.21 Factors that may lead to faster desaturation include: younger age (less than 24 months), and a respiratory indication for intubation.7 Additionally, in 2015, Lee et al completed a prospective study analyzing the national airway registry for children and found that the higher number of intubation attempts was associated with younger age and first provider training level. Those patients who had more than three attempts had more significant desaturations (< 80% SpO2) and suffered severe adverse events (cardiac arrest, hypotension requiring treatment) both with P < 0.001.54,56 However, Rinderknecht et al found evidence to suggest these adverse outcomes were a result of the cumulative duration of laryngoscopy attempts, rather than the number themselves.57 Based on this, they suggested limiting laryngoscopy attempts to 45 seconds.7
Pretreatment
The purpose of pretreatment medications is to prevent potential complications of the intubation itself. However, these agents are controversial and no longer are recommended for routine use. A surveillance study performed by Pallin et al over a 10-year period (2002-2012) showed that use of premedications is on the downward trend, reaching less than 30% by the last year. In the pediatric population, a good example is atropine, which has been a traditional pretreatment agent to prevent bradycardia. Bradycardia commonly occurs during pediatric intubations for multiple reasons including, most commonly, hypoxia, as well as vagal response to laryngoscopy or is iatrogenic secondary to induction medications such as succinylcholine or fentanyl. Prophylactic doses of atropine have been used to hinder this response. Currently, there is no evidence that supports the use of atropine in this way; it has not been shown to improve survival or prevent cardiac arrest. The evidence is varied as to whether pre-intubation atropine decreases arrhythmias or post-intubation shock.56,58 In previous guidelines, a minimal dose of pre-intubation atropine was recommended. However, the 2015 American Heart Association Guidelines on Pediatric Advanced Life Support (PALS) do not recommend the routine use of atropine as a pre-intubation medication in critically ill infants and children. The guidelines state that in situations in which the child is at high risk for bradycardia (i.e., a pure vagally mediated bradycardia), a dose of 0.02 mg/kg with no minimum dose of atropine can be considered.58
Paralysis With Induction
Induction agents for intubation serve to provide amnesia, anesthesia, and analgesia to facilitate passage of the ETT. The ideal medication would render the patient unconscious and unresponsive quickly, all while providing amnesia and pain control with limited side effects. A perfect medication with the above properties does not exist, and an induction agent needs to be selected carefully based on the clinical scenario. Table 4 summarizes the most commonly used induction agents, risks and benefits of their use, and ideal situations in which to use them.
Table 4.Induction Agents to Provide Sedation During Rapid Sequence Induction |
||||||
|
Medication |
Induction Dose |
Onset |
Duration |
Benefits |
Risks |
Ideal Patient Population |
|
Methohexital |
1.5 mg/kg |
< 60 sec |
10 min |
(+) amnesia (+) sedation Dose-dependent ↓ in cerebral metabolic O2 & ICP |
(-) analgesia Central respiratory ↓, venodilation, myocardial depression, bronchospasm |
Euvolemic, normotensive patient |
|
Ketamine |
1.5 mg/kg |
< 60 sec |
20 min |
(+) amnesia (+) sedation (+) anesthesia
Releases catecholamines, ↑ sympathetic nervous system, HR & BP Relaxes bronchial smooth muscle |
Hallucinations may occur on emergence from ketamine, more common in the adult population |
Asthma Hypotensive, hypovolemic, unstable patients, sepsis patients |
|
Propofol |
1.5 mg/kg |
< 60 sec |
10 min |
(+) amnesia (+) sedation Decreases ICP Causes hypotension via vasodilation |
(-) anesthesia Should be used cautiously in hemodynamically unstable patients |
Euvolemic, normotensive patient
Asthma Status epilepticus |
|
Etomidate |
0.3 mg/kg |
< 60 sec |
10 min |
(+) amnesia (+) sedation (+) anesthesia Hemodynamic stability ↓ ICP ↓ cerebral O2 req No histamine release |
Not FDA approved for children, but many case reports demonstrate safety and effectiveness in children May cause transient adrenal suppression use with caution in children with sepsis or major trauma |
Patients w/↑ ICP
Asthma |
|
Midazolam |
0.1 mg/kg to |
60 to 90 sec; up to 2 to 3 min |
15 to 30 min |
(++) amnesia (+) sedation (+) anxiolysis |
(-) anesthesia Dose dependent ↓ in SVR, myocardial depression |
Poor induction agent for RSI secondary to slow onset |
|
Fentanyl |
2 to 3 mcg/kg |
< 60 sec |
30 min |
(+) sedation (+) anesthesia |
Respiratory depression, apnea, hypotension |
Increased ICP cardiovascular disease |
|
ICP: intracranial pressure; HR: heart rate; BP: blood pressure; SVR: systemic vascular resistance; RSI: rapid sequence induction. Adapted from: Walls R, Murphy M. Identification of the Difficult and Failed Airway. In: Manual of Emergency Airway Management. 4th ed. Philadelphia, PA: Wolters Kluwer/Lippincott Williams & Wilkins Health; 2012: 9-20. |
||||||
Sedation medications often are followed quickly by neuromuscular blocking agents (NMBA) to optimize conditions of ETT by providing muscle relaxation and inhibition of normal protective reflexes. The ideal medication would have rapid onset of action, short duration of action, and no significant side effects. These medications often are combined with the above induction agents to provide amnesia, pain control, sedation, and paralysis during RSI. Table 5 summarizes the most common NMBAs and their respective properties.
Table 5.Neuromuscular Blockers and Their Properties |
||||||
|
Medication |
Induction Dose |
Onset (sec) |
Duration (min) |
Benefits |
Risks |
Ideal Patient Population |
|
Succinylcholine (Sch) |
1.5 mg/kg |
45 sec |
6-10 min |
Short onset, short duration of action, well-known side effect profile |
Bradycardia fasciculations, hyperkalemia, Should not be used in patients with burns, crush injuries, denervation, severe infection, or a family history of malignant hyperthermia; excluded in children with myopathies and neurodegenerative disease |
Do not use in children with possible myopathies or concerns for degenerative disease |
|
Rocuronium (Roc) |
1 mg/kg |
60 sec |
60 min |
Can be given in setting of hyperkalemia |
Prolonged duration of action |
Preferred agent when Sch is contraindicated |
|
Vecuronium |
0.08 to 0.1 mg/kg/dose; repeat as needed; higher initial dose (0.15 to 0.2 mg/kg) may be required |
90 sec |
60-75 min |
Can be given in setting of hyperkalemia |
Prolonged duration of action |
Preferred when Sch and Roc are not available |
|
Adapted from: Walls R, Murphy M. Identification of the Difficult and Failed Airway. In: Manual of Emergency Airway Management. 4th ed. Philadelphia, PA: Wolters Kluwer/Lippincott Williams & Wilkins Health; 2012: 9-20. |
||||||
Succinylcholine has been associated rarely with instances of life-threatening malignant hyperpyrexia and reports of rare, but often fatal, hyperkalemic cardiac arrests in young boys with undiagnosed muscular dystrophy. As a result of these reports, in 1994, the FDA recommended that “the use of succinylcholine in children should be reserved for emergency intubation and instances where immediate securing of the airway is necessary, e.g. laryngospasm, difficult airway, full stomach, or for i.m. use when a suitable vein is inaccessible.” Since the publication of this recommendation, the use of succinylcholine in routine anaesthesia in children has been limited with one series showing its use at 0.7%.59
There are situations in which patients are not paralyzed for intubation, and there is evidence supporting higher first-attempt success rates when the patient is adequately sedated and paralyzed. In a 2016 study by Pallin et al, there was a 3.4 times higher success rate when RSI was completed with a sedative and NMBA compared to deep sedation alone. Additionally, multiple prospective studies have demonstrated lower complication rates in intubations using NMBA.49,60-63
Protection and Positioning
As discussed earlier, the pediatric anatomy itself can occlude the airway, which could be worsened in ill patients with airway inflammation. A shoulder roll placed beneath the shoulders often is enough to maintain the neck in a neutral position and align the airway.6 Chin-lift also can be used to create anterior placement of the atlanto-occipital joint; this is also called the “sniffing position.” Always remember to use c-spine precautions if needed; in those circumstances, the jaw thrust maneuver also can be used to relieve mild airway obstruction.64
Placement With Proof
The act of intubating is methodical and can be described in the step-by-step list below, independent of the cause of respiratory compromise.
- Raise the head of the bed to a position of comfort for the operator.
- The positioning of the head needs to be optimal, all while protecting the cervical spine (small shoulder roll placed to align the external auditory meatus with the sternal notch works in all ages).
- Preoxygenate, prepare supplies, and suction the airway.
- Holding the laryngoscope blade in the left hand, insert the blade.
- Open the mouth wide with the right hand while sliding the blade down the tongue to identify the epiglottis.
- Gently lift the mandible upward as you advance the blade and the cords should fall into view.
- Gently insert cuffed tube.
- Confirm placement with auscultation, EtCO2 device, and finally with chest X-ray when appropriate.
- Mechanical ventilation is complicated and beyond the scope of this article, but as a rule, the ventilation rate and tidal volume should be titrated to maintain an EtCO2 reading of 35-45 mmHg and a tidal volume of 6-8 cc/kg.
The gold standard to verify ETT placement is chest X-ray demonstrating the tip of the ET tube 2 cm above the carina.22,64 However, obtaining a prompt chest X-ray is not always realistic in all emergent situations, so the 2010 AHA guidelines recommend the use of EtCO2 to confirm ET tube placement and to measure the quality of chest compressions and ROSC. However, there are no specific values to guide therapy and no pediatric evidence that demonstrates monitoring EtCO2 during a code arrest improves outcomes.65 There is a pediatric-specific device for measuring EtCO2 for patients weighing less than 15 kg; the adult device should be used on those weighing more than 15 kg.12
Post-intubation Care
Once ETT placement has been verified and ventilation is adequate, the tube should be secured and position protected. Medications should be given to provide adequate sedation and, if needed, paralysis. It is important to remember that too much oxygen can be detrimental long term. Some observational studies in children have demonstrated normoxemia (defined as PaO2 between 60 and 300 mmHg) compared to hyperoxemia (> 300 mmHg) after ROSC was associated with improved survival and discharge from the pediatric intensive care unit.65 The 2015 PALS guidelines recommend that rescuers target normoxemia after ROSC with O2 saturations of ≥ 94%.66 A blood gas should be obtained at least one hour after intubation with ventilator settings being adjusted accordingly.
Pitfalls in Management
Any time a procedure is completed, it is important to know and anticipate the potential complications.
Can’t Intubate, Can’t Ventilate
In the rare circumstance that a provider is unable to intubate or ventilate a pediatric patient, an emergent surgical airway may be necessary. This would apply to the critical patient in whom ETT and the use of supraglottic airway devices have been unsuccessful, and BMV is not sufficient to maintain oxygen saturations. As previously discussed, the cricothyroid membrane is virtually nonexistent until 10 years of age, making a needle cricothyroidotomy the preferred intervention in this young age group. An ear, nose, and throat specialist always should be involved in these cases, if possible. The cricothyroidotomy may be performed with a 14-, 16-, or 18-gauge cannula in the cricothyroid space, aiming caudally. Extreme caution should be taken during needle placement, as perforation of the posterior tracheal wall is a common complication. Appropriate placement may be confirmed by air aspiration. Subsequently, the catheter may be connected to a low- or high-pressure oxygen delivery system, depending on the patient’s needs. Because this is an emergent procedure, it is designed to be used only for a short period of time. If the patient is suspected to need airway support for a long period of time, consultation with a specialist for possible conversion to a tracheotomy should be considered.67,68
SUMMARY
There are many anatomic and physiologic differences between pediatric and adult airways. These differences significantly affect the approach to diagnosis and management of the pediatric patient in respiratory distress. The clinical utilization of the PAT is unique to the pediatric population and is an invaluable tool to identify the critical patient in respiratory distress or pending respiratory failure in need of emergency intervention. RSI in the pediatric patient is uncommon, but a necessary skill of the emergency physician.
REFERENCES
- Weiss A, Wier L, Stocks C, Blanchard J. Overview of Emergency Department Visits in the United States, 2011: Statistical Brief #174. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville, MD: Agency for Healthcare Research and Quality; 2006-2014.
- National Safety Council. Injury Facts. Available at: http://www.nsc.org/learn/safety-knowledge/Pages/injury-facts.aspx. Accessed Jan. 1, 2017.
- El Halal MG, Barbieri E, Filho RM, et al. Admission source and mortality in a pediatric intensive care unit. Indian J Crit Care Med 2012;16:81-86.
- Society of Critical Care Medicine. Critical Care Statistics. Available at: http://www.sccm.org/Communications/Pages/CriticalCareStats.aspx. Accessed Jan. 1, 2017.
- Luten RC, Mick NW. Differentiating Aspects of the Pediatric Airway. In: Manual of Emergency Airway Management. 4th ed. Philadelphia, PA: Wolters Kluwer/Lippincott Williams & Wilkins Health; 2012.
- Harless J, Ramaiah R, Bhananker SM. Pediatric airway management. Int J Crit Illn Inj Sci 2014;4:65-70.
- Walls R, Murphy M. Identification of the Difficult and Failed Airway. In: Manual of Emergency Airway Management. 4th ed. Philadelphia, PA: Wolters Kluwer/Lippincott Williams & Wilkins Health; 2012: 9-20.
- Hudgins PA, Siegel J, Jacob I, Abramowsky CR. The normal pediatric larynx on CT and MR. AJNR Am J Neuroradiol 1997;18:239-245.
- Mortensen A, Lenz K, Abildstrøm H, Lauritsen TL. Anesthetizing the obese child. Paediatr Anesth 2011;6:623-629.
- Reuter S, Moser C, Baack M. Respiratory distress in the newborn. Pediatr Rev 2014;35:417-428.
- Hammer J. Acute respiratory failure in children. Paediatr Respir Rev 2013;14:64-69.
- Goldberg L, Pacheco G, Woolridge D. Comprehensive overview of pediatric airway management. Scientific American Emergency Medicine. April 2017.
- Nitu ME, Eigen H. Respiratory failure. Pediatr Rev 2009;30:470-477.
- Marcdante KJ, Kliegman RM. Nelson Essentials of Pediatrics. Philadelphia, PA: Elsevier, Saunders; 2015.
- Vo P, Kharasch V. Respiratory failure. Pediatr Rev 2011;35:476-484.
- Brownstein D. Pediatric Education for Prehospital Professionals. Sudbury, MA: Jones and Bartlett; 2006.
- Horeczko T, Enriquez B, McGrath NE, et al. The Pediatric Assessment Triangle: Accuracy of its application by nurses in the triage of children. J Emerg Nurs 2013;39:182-189.
- Dieckmann RA, Brownstein D, Gausche-Hill M. The Pediatric Assessment Triangle: A novel approach for the rapid evaluation of children. Pediatr Emerg Care 2010;26:312-315.
- Beebe RWO, Funk DL. Fundamentals of Emergency Care. Albany, NY: Delmar; 2001.
- Forgacs P. The functional basis of pulmonary sounds. Chest 1978;73:399-405.
- Choo KJ, Simons FE, Sheikh A. Glucocorticoids for the treatment of anaphylaxis. Cochrane Database Syst Rev 2012 Apr 18; (4):CD007596.
- Langley EW, Gigante J. Anaphylaxis, urticaria, and angioedema. Pediatr Rev 2013;34:247-257.
- Simons FE, Sheikh A. Anaphylaxis: The acute episode and beyond. BMJ 2013;346:f602. doi:10.1136/bmj.f602.
- Richards AM. Pediatric respiratory emergencies. Emerg Med Clin North Am 2016;34:77-96.
- Colavita L, Gelli C, Pecorari L, Peroni DG. A history of recurrent wheezing can delay the diagnosis of foreign body aspiration in a paediatric emergency department. BMJ Case Rep 2015; Sep. 8. pii: bcr2015211946. doi: 10.1136/bcr-2015-211946.
- Rovin JD, Rodgers BM. Pediatric foreign body aspiration. Pediatr Rev 2000;21:86-90.
- Yang C, Hua R, Xu K, et al. The role of 3D computed tomography (CT) imaging in the diagnosis of foreign body aspiration in children. Eur Rev Med Pharmacol Sci 2015;19:265-273.
- Sultan TA, van As AB. Review of tracheobronchial foreign body aspiration in the South African paediatric age group. J Thorac Dis 2016;8:3787-3796.
- Virbalas J, Smith L. Upper airway obstruction. Pediatr Rev 2015;36:62-72.
- D’agostino J. Pediatric Airway nightmares. Emerg Med Clin North Am 2010;28:119-126.
- Russell KF, Liang Y, O’Gorman K, et al. Glucocorticoids for croup. Cochrane Database Syst Rev 2011 Jan 19;(1): CD001955. doi: 10.1002/14651858.CD001955.pub3.
- Mandal A, Kabra SK, Lodha R. Upper airway obstruction in children. Indian J Pediatr 2015;82:737-744.
- Moraa I, Sturman N, McGuire T, van Driel ML. Heliox for croup in children. Cochrane Database Syst Rev 2013;12:CD006822. doi: 10.1002/14651858.CD006822.pub4.
- National Institutes of Health. National Institute on Deafness and Other Communication Disorders. Recurrent Respiratory Papillomatosis or Laryngeal Papillomatosis. Available at: https://www.nidcd.nih.gov/health/recurrent-respiratory-papillomatosis. Accessed April 1, 2017.
- Law JA, Broemling N, Cooper RM, et al. The difficult airway with recommendations for management – Part 1 – Difficult tracheal intubation encountered in an unconscious/induced patient. Can J Anaesthes 2013;60:1089-1118.
- Hinkelbein J, Genzwuerker HV, Fiedler F. Detection of a systolic pressure threshold for reliable readings in pulse oximetry. Resuscitation 2005;64:315-319.
- Macleod DB, Cortinez LI, Keifer JC, et al. The desaturation response time of finger pulse oximeters during mild hypothermia. Anesthesia 2005;60:65-71.
- Brown C, Carleton S. Supplemental Oxygen. In: Manual of Emergency Airway Management. 3rd ed. Philadelphia: Wolters Kluwer/Lippincott Williams & Wilkins Health; 2012:46-51.
- Chisti MJ, Salam MA, Smith JH, et al. Bubble continuous positive airway pressure for children with severe pneumonia and hypoxaemia in Bangladesh: An open, randomised controlled trial. Lancet 2015;386:1057-1065.
- Mikalsen IB, Davis P, Øymar K. High flow nasal cannula in children: A literature review. Scand J Trauma Resusc Emerg Med 2016;24:93.
- Kelly GS, Simon HK, Sturm JJ. High-flow nasal cannula use in children with respiratory distress in the emergency department. Pediatr Emerg Care 2013;29:888-892.
- Salamone FN, Bobbitt DB, Myer CM, et al. Bacterial tracheitis reexamined: Is there a less severe manifestation? Otolaryngol Head Neck Surg 2004;131:871-876.
- Andersen LW, Raymond TT, Berg RA, et al. Association between tracheal intubation during pediatric in-hospital cardiac arrest and survival. JAMA 2016;316:1786-1797.
- Tucker J, Coussa M. Pediatric rapid sequence intubation. Pediatr Emerg Med Rep 2009;14:1-14.
- Raj D, Luginbuehl I. Managing the difficult airway in the syndromic child. Continuing Education in Anaesthesia, Critical Care & Pain 2014;15:7-13.
- Belanger J, Kossick M. Methods of identifying and managing the difficult airway in the pediatric population. AANA J 2015;83:35-41.
- Heinrich S, Birkholz T, Ihmsen H, et al. Incidence and predictors of difficulat laryngoscopy in 11,219 pediatric anesthesia procedures. Paediatr Anaesth 2012;22:729-736.
- Lee A, Herkner H, Hovhannisyan K, Pace NL. Airway physical examination tests for detection of difficult airway management in apparently normal patients. Cochrane Database Syst Rev 2010; CD008874. doi:10.1002/14651858.cd008874.
- Tayal VS, Riggs RW, Marx JA, et al. Rapid-sequence intubation at an emergency medicine residency: Success rate and adverse events during a two-year period. Acad Emerg Med 1999;6:31-37.
- Shi F, Xiao Y, Xiong W, et al. Cuffed versus uncuffed endotracheal tubes in children: A meta-analysis. J Anesth 2015;30:3-11.
- Luten R, Wears RL, Broselow J, et al. Managing the unique size-related issues of pediatric resuscitation: Reducing cognitive load with resuscitation aids. Acad Emerg Med 2002;9:840-847.
- Singh A, Frenkel O. Evidence-based emergency management of the pediatric airway. Pediatr Emerg Med Pract 2013;10:1-28.
- Saracoglu KT, Eti Z, Kavas AD, Umuroglu T. Straight video blades are advantageious than curved blades in siumulated pediatric difficult intubation. Paediatr Anaesth 2014;24:297-302.
- Pallin DJ, Dwyer RC, Walls RM, Brown CA; NEAR III Investigators. Techniques and trends, success rates, and adverse events in emergency department pediatric intubations: A report from the National Emergency Airway Registry. Ann Emerg Med 2016;67:610-615.
- Balaban O, Hakim Walia H, et al. A comparison of direct laryngoscopy and videolaryngoscopy for endotraceheal intubation by inexperienced users: A pediatric Manikin study. Pediatr Emerg Care 2017; Jun. 6. doi: 10.1097/PEC.0000000000001198. [Epub ahead of print[].
- Lee JH, Turner DA, Kamat P, et al. The number of tracheal intubation attempts matters! A prospective multi-institutional pediatric observational study. BMC Pediatr 2016;16:58.
- Rinderknecht AS, Mittiga MR, Meinzen-Derr J, et al. Factors associated with oxyhemoglobin desaturation during rapid sequence intubation in a pediatric emergency department: Findings from multivariable analyses of video review data. Acad Emerg Med 2015;22:431-440.
- Jones P, Dauger S, Denjoy I, et al. The effect of atropine on rhythm and conduction disturbances during 322 critical care intubations. Pediatr Crit Care Med 2013;14:e289-297.
- Tarquinio KM, Howell JD, Montgomery V, et al. Current medication practice and tracheal intubation safety outcomes from a prospective multicenter observational cohort study. Pediatr Crit Care Med 2015;16:210-218.
- Naguib M, Samarkandi AH, El-Din ME, et al. The dose of succinylcholine required for excellent endotracheal intubating conditions. Anesth Analg 2006;102:151-155.
- Sagarin MJ, Chiang V, Sakles JC, et al. Rapid sequence intubation for pediatric emergency airway management. Pediatr Emerg Care 2002;18:417-423.
- Li J, Murphy-Lavoie H, Bugas C, et al. Complications of emergency intubation with and without paralysis. Am J Emerg Med 1999;17:141-143.
- Gnauck K, Lungo JB, Scalzo A, et al. Emergency intubation of the pediatric medical patient: Use of anesthetic agents in the emergency department. Ann Emerg Med 1994;23:1242-1247.
- Fastle RK, Roback MG, et al. Pediatric rapid sequence intubation. Pediatr Emerg Care 2004;20:651-655.
- de Caen AR, Berg MD, Chameides L, et al. Part 12: Pediatric Advanced Life Support: 2015 American Heart Association Guidelines Update for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2015;132(18 Suppl 2):S526-542.
- Ferguson LP, Durward A, Tibby SM. Relationship between arterial partial oxygen pressure after resuscitation from cardiac arrest and mortality in children. Circulation 2012;126:335-342.
- Jagannathan N, Sohn L, Fiadjoe JE. Paediatric difficult airway management: What every anaesthetist should know! Br J Anaesth 2016;117(Suppl 1):i3-i5.
- Black AE, Flynn PE, Smith HL, et al. Development of a guideline for the management of the unanticipated difficult airway in pediatric practice. Pediatr Anesth 2015;25:346-362.
Pediatric patients frequently present with respiratory complaints. Fortunately, most children respond well to simple medical interventions. Understanding a child's anatomic and physiologic differences is critical to effectively preventing respiratory failure and stabilizing a child when it occurs.
Subscribe Now for Access
You have reached your article limit for the month. We hope you found our articles both enjoyable and insightful. For information on new subscriptions, product trials, alternative billing arrangements or group and site discounts please call 800-688-2421. We look forward to having you as a long-term member of the Relias Media community.