Arterial Ischemic Stroke Prevention and Risk Factor Management
AUTHORS
Michael P. Lerario, MD, Department of Neurology, Weill Cornell Medical College, New York; New York-Presbyterian/Queens Hospital, Flushing, NY
Alan Z. Segal, MD, Department of Neurology, Weill Cornell Medical College, New York
PEER REVIEWER
David A. Decker, MD, Director, Comprehensive Stroke Center, Florida Hospital Tampa, Tampa, FL
To reveal any potential bias in this publication, and in accordance with Accreditation Council for Continuing Medical Education guidelines, Dr. Wise (editor) reports he is on the speakers bureau for the Medicines Company. Dr. Lerario (author), Dr. Segal (author), Dr. Decker (peer reviewer), Ms. Coplin (executive editor), and Mr. Springston (associate managing editor) report no financial relationships relevant to this field of study.
EXECUTIVE SUMMARY
Stroke affects nearly 800,000 people annually in the United States and is the fifth most common cause of death and a leading cause of long-term disability.
- Modifiable risk factors for stroke include hypertension, hypercholesterolemia, obstructive sleep apnea, and diabetes.
- The ABCD2 score is a commonly used scale that can help stratify the risk of subsequent stroke in patients following TIA.
- In the management of atrial fibrillation, novel oral anticoagulants have been shown to be at least noninferior to warfarin for ischemic stroke prevention and better tolerated in terms of intracranial bleeding risk.
- Although symptomatic cervical carotid stenosis is amenable to revascularization, stenting of intracranial atherosclerosis has proven harmful in clinical trials.
Stroke is a common problem, affecting nearly 800,000 people annually in the United States.1 Domestically, it is the fifth most common cause of death and a leading cause of significant long-term disability.1 Stroke costs the United States an estimated $34 billion each year.1 Given this extraordinary burden on the health of the American population, appropriate stroke prevention measures could dramatically improve our quality and length of life.
The widespread institution of screening, risk factor modification, and treatment of known cardiac and cerebrovascular disease is paramount to the health of our nation. The following article discusses the best evidence-based practice for stroke diagnosis, prevention, and risk factor management. Where strong data are absent, usual practice and expert consensus are accepted and noted as such.
Stroke Epidemiology
There are nearly 800,000 strokes in the United States each year, with 130,000 associated deaths.1,2 Although stroke is the second leading cause of death worldwide, it is only the fifth most likely cause in the United States.1,2 Because of the major disability associated with stroke, the overall cost to this country is substantial. Up to 30% of patients who survive a stroke will require institutional care.2 Mortality from stroke varies according to ethnicity, with blacks twice as likely to suffer a stroke and more likely to die compared to whites.2 The “stroke belt” in the southeastern United States has the highest national stroke mortality.2 Stroke risk increases with age, but 34% of stroke affects individuals younger than 65 years of age.2 The overall stroke incidence is expected to rise in the near future as the population ages, despite recent advances in risk factor reduction, which has reduced the per capita incidence of strokes. There are gender differences in stroke rates as well.Women are 50% more likely than men to have a stroke, particularly due to a high incidence in white elderly females.2
Modifiable Stroke Risk Factors
Hypertension is the most important modifiable stroke risk factor. Multiple trials have shown the benefit of blood pressure control, including many different agents such as angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, calcium channel blockers, and diuretics.3 The choice of agent is less crucial than the successful lowering of blood pressure. In general, blood pressure treatment should be targeted to a normal blood pressure of < 140/90 mmHg.3 However, recent evidence suggests a more aggressive goal of systolic < 120 mmHg may be beneficial for the primary prevention of cardiovascular events.4 Although these data applied to a composite outcome of stroke, myocardial infarction, heart failure, and vascular death, they confirm that “pre-hypertension” (diastolic 80-90 and systolic 120-140) contributes to increased stroke risk.
Hypercholesterolemia is another important stroke risk factor. Low-density liporprotein (LDL) cholesterol in patients with a prior stroke should be < 70 mg/dL. Cholesterol lowering is particularly important in patients with a prior history of atherosclerotic disease, in which case the American Heart Association (AHA) recommends titrating statins to the maximum tolerated dosage (high-intensity statin) rather than specific LDL goals.5 Large cohorts of patients with prior myocardial infarction, such as the Heart Protection Study, have shown that control of lipids not only prevents future heart attack but specifically lowers stroke rates by about 25%.6
Obstructive sleep apnea (OSA) approximately doubles the risk of stroke when compared to controls, and stroke rates increase steadily with OSA severity.7 The mechanism by which OSA results in cerebral embolism is not entirely certain; however, it is known that sleep-disordered breathing increases the development of atrial fibrillation, a potent stroke risk factor.8 The treatment for OSA is quite effective. Continuous positive airway pressure (CPAP) therapy has been shown to be effective in reducing stroke risk to equal patients with no history of sleep-disordered breathing.7
Diabetes is known to confer excess risk of stroke independent of blood pressure.9 Specific stroke mechanisms, such as large artery atherosclerotic disease or small vessel ischemia, are associated with poor glucose control.9 Despite stroke being a known vascular complication of diabetes, tight glucose control never has been shown to prevent stroke conclusively, due to a lack of randomized data. Nevertheless, given the multiple health risks associated with uncontrolled diabetes, it is recommended to avoid hyperglycemia in patients who are at risk for stroke.3
Transient Ischemic Attack
Transient ischemic attack (TIA) is a well-known and relatively common risk factor for stroke. An estimated 240,000 TIAs occur each year in the United States.10 TIAs are caused by the same mechanisms as ischemic stroke and, therefore, can be an identifiable harbinger of subsequent vascular events.
The definition of TIA has changed in recent years from time-based to tissue-based criteria. TIA originally was defined clinically by the acute onset of neurological deficits that resolved completely within 24 hours. However, this time limit was arbitrarily selected and may include many cases of patients with evidence of infarction on MRI, despite the resolution of symptoms.11 The majority of patients with MRI-negative TIA have symptoms that last less than one hour.11,12 Furthermore, patients with temporary symptoms and infarction on MRI have a higher risk of recurrent stroke.11,13 Due to these factors, it has since been proposed that TIA should be defined by the absence of acute infarction, based on neuropathologic, neuroimaging, or clinical evidence of permanent injury.12 Such a tissue-based definition of TIA has reduced the estimates of annual incidence of TIA by 33% but has increased the accuracy of diagnosis, as many of these events are now correctly defined as stroke instead.14 An accepted definition of TIA in current practice would be symptoms lasting less than one hour and without evidence of acute infarct on imaging.
Patients who have suffered a TIA require urgent evaluation due to the high risk of stroke following transient cerebral ischemia. The ABCD2 score is a commonly used scale that can help stratify this risk of subsequent stroke. (See Table 1.) The 48-hour stroke rates for patients with low-risk (0-3), moderate-risk (4-5), and high-risk (6-7) scores on this TIA prognostication scale are 1%, 4%, and 8% , respectively.15 Although these rates of recurrent stroke have decreased in patients with contemporary risk factor management, the ABCD2 score remains a useful tool for risk stratification after TIA.16
Table 1. ABCD2 Score |
|
|
A |
Age > 60 years: 1 |
|
B |
Blood pressure > 140/90: 1 |
|
C |
Clinical features
|
|
D |
Diabetes: 1 |
|
D |
Duration
|
Although not its original intent, clinicians use the ABCD2 score to aid in the triaging of patients presenting to the emergency department with TIA. Typically, patients with a score of 4 or more are admitted to the hospital. This system can be used safely to help triage patients for expedited inpatient evaluation, as opposed to outpatient follow-up, and reduce hospitalizations.17 The ABCD2 scoring system may be falling out of favor since some recent reports have found that it overestimates the risk of stroke,17 and it may not be able to discriminate a true vascular event from a stroke mimic, such as migraine aura, seizure, or peripheral neuropathy, confidnetly.18 Furthermore, the ABCD2 does not assess for high-risk mechanisms of TIA, such as atrial fibrillation or carotid stenosis, which may require a specific urgent intervention (e.g., anticoagulation or a revascularization procedure). Therefore, it is recommended that TIA patients undergo urgent neurovascular imaging, with either ultrasound, MR, or CT angiography, and obtain a cardiac evaluation, including 12-lead electrocardiogram and transthoracic echocardiogram at a minimum. This evaluation typically occurs as an inpatient for most cases of true TIA. If a patient is discharged for outpatient workup, the AHA/American Stroke Association (ASA) recommends that this evaluation be completed within 48 hours.12 In such instances, a specialized TIA clinic that evaluates these patients on an urgent outpatient basis may be beneficial for reducing early recurrent stroke by as much as 80%.19
Stroke prevention strategies in TIA patients are similar to those with ischemic stroke. Appropriate antithrombotic selection, statin usage, and aggressive risk factor management are key components of an effective secondary stroke prevention regimen. Patients with high-risk TIA (defined as having an ABCD2 score of 4 or more) may benefit from short-term dual antiplatelet agents. In the CHANCE trial, the combination of aspirin 75 mg and clopidogrel 75 mg for three weeks reduced the absolute risk of recurrent stroke by 3.5% compared to aspirin alone, without an increase in major bleeding.20 All the patients recruited for this trial were from mainland China, so a similar study (known as POINT) is being conducted to see if these results translate to improved stroke outcomes in a strictly North American population.
Stroke Mimics
Mimics are non-cerebrovascular disorders that confound a stroke diagnosis by presenting in a similar manner. As many as 20% of patients initially diagnosed with stroke eventually are found to have a mimic.21,22 Common conditions that mimic stroke include seizure with postictal deficits, migraine aura, conversion disorder, encephalopathy from metabolic disturbances, intracranial tumors or infections, hypertensive encephalopathy, Bell’s palsy, transient global amnesia, spinal or nerve disorders, peripheral vertigo, and syncope. The discrimination of a true vascular event from a mimic is important in terms of treatment and follow-up. Whereas TIA is a known risk factor for subsequent stroke, the risk of future vascular events after the diagnosis of a mimic is negligible.23,24 The ABCD2 score may be helpful in making the diagnosis of a non-vascular event. Lower scores on this scale are associated with mimics, rather than true TIAs, and have a low likelihood of future stroke.24 Nevertheless, this differentiation between mimic and true stroke may not be as important in the acute setting, since it has been deemed safe to treat mimics with intravenous (IV) tissue plasminogen activator. The risk of symptomatic intracranial hemorrhage following IV thrombolysis has been demonstrated to be 1% or less.22,25
Stroke Mechanisms
Causes of Stroke. Ischemic stroke, the damage to neuronal tissue as a result of reduced cerebral blood flow, can be a common endpoint due to many conditions. The main causes of stroke are due to either thrombosis or embolism of a cerebral artery. Thrombosis refers to a local occlusive process formed in situ within the artery. The site of obstruction may occur either within a large intra- or extracranial artery (which is typically the result of atherosclerosis) or within a small penetrating artery (which typically is due to chronic vessel changes from hypertension).26,27 On the other hand, embolism refers to thrombus that travels from the site of formation and lodges within distal vessels. The source of embolism either may be a proximal artery, the heart, or paradoxically from the venous system in the case of a patent foramen ovale (PFO).26,27 Embolic strokes tend to cause symptoms that are abrupt and maximal at onset.26 Additionally, embolism often leads to cortical infarction in the cerebral surface of an arterial territory and is more likely to be associated with hemorrhagic conversion.28
TOAST Classification System. There have been many attempts to categorize mechanisms of stroke for clinical and research purposes. A commonly used classification schema for stroke subtype is known as the TOAST system.27 (See Table 2.) The TOAST system has inherent limitations, namely the large number of stroke patients who resultantly are classified as cryptogenic (i.e., having undetermined etiology). The TOAST investigators did not require aggressive diagnostic testing, by today’s standards, prior to categorizing a patient as cryptogenic; for instance, transesophageal echocardiogram and extended arrhythmia monitoring were not required. Nevertheless, it is a useful tool for research purposes and for conceptualizing stroke mechanism in a simplified manner.
Table 2. TOAST Classification System of Stroke Subtypes |
|
|
Stroke Subtype |
Prevalence of Stroke Subtype32 |
|
Large artery atherosclerosis |
15.5% |
|
Cardioembolism |
27.8% |
|
Small vessel occlusion |
18.1% |
|
Stroke of other determined etiology |
4.2% |
|
Stroke of undetermined etiology
|
34.4% |
Lacunar Stroke. Lacunar stroke refers to the pathophysiological, clinical, and radiographic findings observed in small vessel disease. A lacunar stroke is the result of arterial obstruction of a single deep, penetrating vessel that supplies the subcortical structures of the brain, such as the capsule, basal ganglia, thalamus, and paramedian brainstem.26,29 Such arterial obstruction is associated with the pathological changes occurring in response to chronic hypertension or diabetes, including microatheroma or lipohyalinosis.26 Lipohyalinosis refers to the degenerative change in small blood vessels due to the accumulation of lipid within the vessel wall.26
Since the affected arteries are small, the resultant strokes (known as lacunes) also are small. These irregular cavitary lesions typically are < 1.5 centimeters in diameter.30 Radiographically, lacunar strokes appear as small infarcts in typical subcortical structures, with MRI being more sensitive than CT for detecting these lesions.26 (See Figure 1.) The imaging findings of a small, deep infarct in the absence of other possible stroke etiologies is strongly supportive of a lacunar etiology. Larger areas of infarction, known as giant or super lacunes, may imply thrombosis or embolism of the proximal branch from which the penetrating artery arises; for instance, a lenticulostriate territory stroke could result from a nonocclusive embolus to the middle cerebral artery.26 Therefore, subcortical strokes > 1.5 centimeters in size may necessitate a diagnostic evaluation aimed at finding an embolic source of stroke, including echocardiography, telemetry, and noninvasive angiography of the head and neck.
Figure 1. MRI Displaying Typical Imaging Characteristics of Acute Lacunar Infarctions |
|
Note small, deep infarctions in the left paramedian pons (A) and right thalamus (B).
A |
Lacunar infarcts clinically present as one of several classic syndromes, with the following being the most common: pure motor weakness, pure sensory loss, mixed sensorimotor, ataxia hemiparesis, and dysarthria-clumsy hand syndromes.31 The symptoms of lacunar stroke often fluctuate over the acute course of the disease, hence the name “stuttering lacune.” Although lacunar strokes comprise nearly 20% of strokes,32 they have the lowest in-hospital mortality of the subtypes.33
Cardioembolic Stroke. Cerebral embolism occurs when particulate material from a proximal source travels through the arterial system to lodge within a downstream cerebral artery. Emboli to the brain are most often composed of mural thrombi or platelet aggregates and most often travel to the anterior circulation (particularly the middle cerebral artery territory), given that these arteries accept the majority of cerebral blood flow.26 Although an embolic etiology accounts for up to 70% of stroke cases,26 probable or definite evidence of cardiac embolism is demonstrated in only 25-30% of ischemic strokes.32 In other instances, the source of embolus may be a proximal large artery, the venous circulation in the case of a PFO, or unknown (cryptogenic).27 A cardiac source is implied if the emboli result in bilateral infarcts, particularly if cortical and in multiple vascular territories.28 (See Figure 2.) For example, a potential cardioembolic etiology of stroke would be inferred from an MRI demonstrating simultaneously occurring left middle cerebral artery and right posterior cerebral artery acute transcortical infarctions. These emboli tend to be evanescent, and obstruction of an intracranial artery may recanalize spontaneously,34 likely contributing to the higher rate of intracerebral hemorrhage with reperfusion associated with embolic strokes.28
Figure 2. MRI Demonstrating Typical Imaging Characteristics of Cardioembolic Strokes |
|
A-B show acute infarctions in bilateral cerebral hemispheres.
A |
|
C-D show chronic transcortical infarctions in the occipital lobe of the same patient.
C |
There are many potential causes of cerebral cardioembolism. (See Table 3.) Whether a stroke can be attributed to a cardioembolic source requires the identification of a known cardiac risk factor for embolic stroke, as well as the exclusion of other etiologies, such as large artery atherosclerosis or lacunar infarct, based on dedicated neuroimaging.27 The probability of a stroke being attributed to an identified cardiac risk factor depends on how strongly that risk factor is associated with ischemic stroke.27 The most common high-risk sources of cardioembolism include valvular heart disease, the formation of an intracardiac thrombus (e.g., recent myocardial infarction or atrial fibrillation), ventricular or septal aneurysms, and cardiomyopathies. Three specific cardiac sources of stroke, including atrial fibrillation, PFO, and aortic arch atheroma, are discussed in depth below.
Table 3. Potential Sources of Cerebral Cardioembolism 27 |
|
|
High-risk Sources
|
Medium-risk Sources
|
Atrial Fibrillation as a Stroke Risk Factor. Atrial fibrillation is the most common sustained cardiac arrhythmia, occurring in 1-2% of the general population, and is even more prevalent in aging populations.35 Atrial fibrillation can lead to left atrial or atrial appendage thrombus formation, which can serve as a source of embolism. The arrhythmia is a very strong risk factor for stroke, increasing this risk by as much as five times.35 Stroke risk in atrial fibrillation patients can be stratified by using scales such as the CHADS2 and CHA2DS2-VASc scores. (See Tables 4A and 4B.)
Table 4A. The CHA2DS2-VASc Score |
||
|
C |
Congestive heart failure |
1 point |
|
H |
Hypertension |
1 point |
|
A2 |
Age > 75 years Age 65-74 years |
2 points 1 point |
|
D |
Diabetes |
1 point |
|
S2 |
Stroke, transient ischemic attack, or prior embolism Female |
2 points 1 point |
|
VASc |
History of vascular disease |
1 point |
Table 4B. Annual Stroke Risk Stratified by CHA2DS2-VASc Score |
|
|
Score |
Adjusted stroke rate35 |
|
0 |
0 |
|
1 |
1.3% |
|
2 |
2.2% |
|
3 |
3.2% |
|
4 |
4.0% |
|
5 |
6.7% |
|
6 |
9.8% |
|
7 |
9.6% |
|
8 |
6.7% |
|
9 |
15.2% |
PFO as a Stroke Risk Factor. A PFO is a persistent communication between the two atria of the heart, which typically closes after birth. However, in 25% of people, the foramen ovale remains open and could serve as a conduit between the venous and arterial systemic circulations.36 Such a circulatory configuration can lead to the passage of venous thrombotic material into the arterial circulation, potentially resulting in a paradoxical embolus to the brain. The recurrent stroke rates due to this structural defect are relatively low, particularly on medical therapy; however, some studies have shown that a large shunt size or atrial septal aneurysm may increase the risk of stroke.36,37 A PFO may be detected on echocardiography or transcranial Doppler with the injection of agitated saline.
Aortic Arch Atheroma as a Stroke Risk Factor. Aortic arch atheroma is a manifestation of systemic atherosclerosis and may lead to thromboembolism in 21% of patients.38 Most of these thromboembolic events occur within the cerebral vasculature, typically involving small and medium-sized intracranial arteries. The source of embolism is most often a superimposed thrombus on an unstable plaque.39 Since aortic arch atherosclerosis is common in older populations, it is important to identify which plaque features pose the highest risk for causing stroke and, therefore, warrant specific attention. Transesophageal echocardiogram is the optimal test to detect complex aortic arch atheromatous disease, with a 90% sensitivity and specificity. A plaque is considered complex if it is more than 4 mm in thickness, has ulcerated or mobile components, or has a high degree of protrusion into the aortic lumen. The size of the plaque seems to highly correlate with stroke risk, where two-year recurrent stroke rates are 4% or less if the plaque is < 4 mm and 12% if the plaque is > 4 mm.40 Before attributing a stroke to this etiology, first it is important to rule out other sources of cardioembolism, such as atrial fibrillation, as this finding may necessitate a change in medical management to include anticoagulation.
Large Artery Atherosclerosis. Stenosis in the intracranial vessels is associated with a very high recurrent stroke risk, even for patients treated with aggressive medical management. The rate of stroke with cerebral atherosclerotic disease was as high as 15% per year in recent randomized trials.41,42 This risk is doubled in patients with severe intracranial stenosis (70-99%);43 however, any stenosis that is hemodynamically significant (> 50%) poses an increased risk. Intracranial atherosclerosis is more prevalent in certain racial groups, such as Asians, blacks, and hispanics.44 The arteries most likely to be affected by atherosclerosis, in order of descending prevalence, include the vertebral or basilar arteries, the middle cerebral artery, the anterior cerebral artery, and the posterior cerebral artery.45 Intracranial atherosclerosis may cause stroke through several mechanisms. For instance, strokes may be the result of a hemodynamically low-flow state. Alternatively, in situ thrombosis may cause localized occlusion, or there may be distal propagation of clot or “artery-to-artery” embolism.
Extracranial arterial stenosis, primarily found in the internal carotid artery just distal to its bifurcation from the common carotid, is most associated with stroke when the severity of stenosis is > 50%. Stroke risk is higher in the population with > 70% stenosis than in the 50-69% range; however, in patients who have already suffered a stroke (defined as symptomatic carotid stenosis), both groups will require surgical or endovascular intervention.46 Carotid stenosis in asymptomatic patients is considerably more controversial. Intervention is indicated for stenosis > 70%, especially as severity progresses toward 80-90%.47,48 Patients with 90-99% asymptomatic carotid stenosis may have a lower stroke risk than those in lesser groups, due to the gradual formation of extensive collaterals.
There are multiple methods to image carotid stenosis; conventional angiography is the gold standard. (See Figure 3.) Carotid ultrasound is an excellent tool with which to screen and follow carotid stenosis, but most surgeons require an anatomical picture pre-operatively. MR angiography and CT angiography are commonly used for this purpose.
Figure 3. Conventional Angiogram Demonstrating the Treatment for Stenosis of the Extracranial Internal Carotid Artery |
|
A: A calcified atheromatous plaque is demonstrated 2 cm distal to the origin of the left internal carotid artery, resulting in > 90% stenosis. B: Status after the successful angioplasty and stenting of the carotid artery with mild residual luminal stenosis.
A |
Stroke of Other Determined Etiology. Stroke of other determined etiologies comprise < 5% of stroke subtypes under the TOAST classification system.32 There are many specific causes of stroke that do not meet the criteria to be classified as large artery, cardioembolic, or lacunar disease. This includes various rarer causes of stroke, many of which also are more prevalent in younger stroke populations. In the stroke literature, young typically is defined as younger than 45 (or 55) years old.49 See Table 5 for a list of causes of stroke in the young, which includes various nonatherosclerotic angiopathies (e.g., arterial dissection), hematological and genetic conditions (e.g., acquired or congenital hypercoagulable state), and vasculopathy secondary to inflammatory or infectious processes (e.g., primary central nervous system [CNS] vasculitis).49 This discussion will be limited to these three conditions. However, it is important to note that even in a younger age group, traditional vascular risk factors and stroke mechanisms may be responsible for an incident stroke.49 The prevalence of hypertension, diabetes, and dyslipidemia has been increasing in young adults. Therefore, initial, standard neurovascular imaging and cardiac evaluation should be pursued in young stroke patients, prior to ordering burdensome diagnostics for rare etiologies of stroke.
Table 5. Specific Causes and Diagnostic Workup for Rare Etiologies of Stroke in the Young 49 |
|
Infectious Endocarditis: ESR, CBC, TEE, blood cultures Tuberculosis: CSF TB PCR, PPD, CXR Syphilis: RPR, CSF VDRL or FTA-ABS VZV: CSF PCR HIV: serologic testing Bacterial meningitis: CSF culture |
|
Autoimmune Primary CNS angiitis: CSF cells and protein, cerebral angiogram, brain biopsy Systemic vasculitis:
Systemic autoimmune diseases: Focused serum rheumatological panel
Inflammatory bowel disease |
|
Genetic Factor V Leiden Prothrombin gene G20210A mutation MTHFR C677T mutation CADASIL: NOTCH 3 mutation by skin biopsy Fabry’s disease: alpha galactosidase activity MELAS: Serum and CSF lactate, CK, muscle biopsy, mitochrondrial DNA sequencing Neurofibromatosis Sturge-Weber disease |
|
Hematologic Protein C/S deficiency Antithrombin III deficiency APLS: lupus anticoagulant, anti-cardiolipin, and anti-beta-2 glycoprotein I antibodies Hyperhomocysteinemia Sickle cell disease: hemoglobin electropheresis DIC TTP Acquired hypercoagulable state:
|
|
Nonatherosclerotic Angiopathies: Neurovascular Imaging (CTA, MRA, Doppler, DSA) Arterial dissection Fibromuscular dysplasia Moyamoya syndrome Migrainous infarction Reversible cerebral vasoconstriction syndrome |
Cervical Arterial Dissection as a Stroke Risk Factor. Cervical arterial dissections are a rarer cause of stroke, with an annual incidence of two to three per 100,000 and a peak prevalence in the fifth decade of life.50,51 A diagnosis of arterial dissection should be considered in young patients with a history of trauma, particularly if they have ipsilateral neck pain, headache, or a Horner’s syndrome on examination (from disrupted sympathetic fibers running along the carotid artery).50 A dissection results from an intimal tear of the affected artery, allowing for blood to extravasate into a false lumen.51 This ultimately leads to intramural hematoma formation and vessel stenosis, or alternately, embolization from a thrombus formed at the dissection site can cause stroke.51 Dissections can be spontaneous or traumatic from direct pressure to or hyperextension of the neck, or from intense straining, such as that seen with severe coughing bouts. Typically, diagnosis is made with cervical neurovascular imaging. MRI with cross-sectional imaging of the vessel can detect intramural hematoma, and contrast angiography can show crescentic enhancement of the vessel or a progressive tapering of the visualized arterial lumen.50,51 Stroke rates following dissection are variable in the literature and range from 0 to 13%.50
Cancer-related Hypercoagulability as a Stroke Risk Factor. Cancer-related hypercoagulability is an under-appreciated cause of stroke. Patients with cancer make up 10% of the stroke population, and the majority of strokes in these patients occur within six months of cancer diagnosis.52,53 There are several unique mechanisms for ischemic stroke that are observed in patients with active cancer. (See Table 6.) The most common cancers in stroke patients include primary brain tumors and hematological malignancies, as well as lung, breast, prostate, gynecological, and pancreatic cancers.52,53 Patients with adenocarcinoma, systemic metastases, or cryptogenic stroke subtypes seem to be particularly high-risk groups.54,55 Clues to occult cancer in ischemic stroke patients include multiple brain lesions in multiple vascular territories, as well as elevated D-dimer levels.56 Transesophageal echocardiogram is superior to transthoracic studies in detecting a source of cardioembolism in these patients.53,56
Table 6. Ischemic Stroke Mechanisms Specific to Patients with Cancer |
|
Primary CNS Angiitis as a Stroke Risk Factor. Primary CNS angiitis is a rare diagnosis, with an incidence of only 2.4 cases per 1 million person-years.57 The diagnosis is made through the identification of inflammation within small- and medium-sized leptomeningeal and parenchymal arteries within the CNS, without any evidence of systemic involvement. As a result, a brain biopsy including affected areas of the cortex and leptomeninges is required to clinch a diagnosis of definite vasculitis as well as to rule out alternative diagnoses, such as demyelinating, infectious, or neoplastic diseases.58 The diagnosis may be supported with the presence of an inflammatory cerebrospinal fluid (CSF) profile and angiography demonstrating alternating arterial stenoses and dilatations, although both have relatively low sensitivity and positive predictive values.57,59 ESR and CRP are often normal.57,59 Since the disease may present with protean manifestations, a standardized diagnostic criteria is difficult, but a recommended approach to diagnostic evaluation is presented in Table 7. A diagnosis of primary CNS angiitis should be suspected if patients present with constitutional symptoms, atypical headache, or progressive cognitive impairment, which are all common clinical manifestations of the disease.57 Imaging studies may demonstrate diverse-appearing lesions, which range from single to multiple, ischemic to hemorrhagic (or both), and which additionally may display contrast enhancement or mass-like qualities.57 The diagnosis is often missed due to the rarity of the disease, the multidisciplinary diagnostic evaluation required, and the variability in terms of the clinical symptomatology, acuity of onset, location of CNS involvement, and imaging characteristics.
Table 7. Suggested Diagnostic Evaluation for Primary CNS Angiitis |
|
Cryptogenic. The term cryptogenic refers to those strokes of uncertain etiology. Unfortunately, approximately 23-40% of strokes are of a cryptogenic mechanism.32,60 The prevalence of cryptogenic stroke is higher in younger patient populations, where traditional vascular risk factors may not play as large of a role.60
Before a patient is confirmed as cryptogenic, a routine diagnostic evaluation for stroke mechanisms should be undertaken to rule out large artery, cardioembolic, or small vessel disease. This workup standardly includes dedicated neurovascular imaging and cardiac testing.60,61 Cervical and cranial noninvasive angiography can be accomplished through the use of either MRI or contrast CT technologies; transcranial and carotid dopplers could replace these studies if patients have contraindications to MRI or iodinated contrast. Cardiac evaluation should include a 12-lead ECG, inpatient telemetry for at least 24 hours, and echocardiography. The yield of standard transthoracic echocardiography is rather low for detecting high-risk stroke mechanisms, so if a patient is highly suspected to harbor an occult cardiac source of embolism, transesophageal echocardiography should be performed.60
The prevailing thought is that a majority of these cryptogenic strokes are from occult embolic sources, hence the recently coined term “embolic stroke of undetermined source” (ESUS).61 Potential nontraditional embolic etiologies of cryptogenic stroke include covert paroxysmal atrial fibrillation, nonstenosing (< 50%) atherosclerotic disease, and occult hypercoagulable states.60,61 (See Table 8.) There is more and more emerging evidence that links occult embolic mechanisms to cryptogenic stroke. Previously thought minor cardiac risk factors now have been associated with stroke, independent of atrial fibrillation, including supraventricular tachycardia,62 elevated BNP,63 abnormal P wave morphology,64 and left atrial dilatation on echocardiography.65 Many patients with cryptogenic stroke who undergo prolonged arrhythmia monitoring are eventually discovered to have covert paroxysmal atrial fibrillation.66,67 Additionally, arterial stenosis < 50% has been associated with ischemic stroke68 and cryptogenic stroke,69 and can be a source of atheroembolism.
Table 8. Potential Etiologies of Cryptogenic Stroke |
|
Covert paroxysmal atrial fibrillation |
|
Cancer-associated hypercoagulable state, including marantic endocarditis |
|
Nonstenosing (< 50%) plaques of large cervical or intracranial arteries |
|
Aortic arch atheroma |
|
Paradoxical embolism from PFO, atrial septal defect, or pulmonary arteriovenous fistula |
|
Minor-risk potential cardioembolic sources
|
|
Branch occlusive disease |
The reported rates of recurrent stroke in cryptogenic stroke and ESUS vary (due to differences in diagnostic criteria, age, and a non-standardized medical treatment) but range from 3-14% per year.70 In a recent report from a prospective registry of ESUS patients, there was a cumulative probability of stroke recurrence of 29% at a mean of 30.5 months.71 This rate of stroke recurrence was similar to cardioembolic strokes but higher than noncardioembolic strokes.
Secondary Stroke Prevention Methods
Management of Lacunar Stroke. As lacunar strokes are often the result of pathophysiological changes from chronic hypertension, aggressive monitoring and control of this risk factor is integral to the secondary stroke prevention of small vessel disease. Although blood pressure targets need to be individualized based on patients’ comorbidities, current guideline recommendations advise a target pressure < 140/90 mmHg in patients who have suffered strokes.3 However, newer evidence from the SPS3 trial suggests that there may be some benefit from more strict blood pressure control (systolic < 130 mmHg) in patients with lacunar stroke.72 SPS3 showed a strong trend toward reduction in the primary outcome of all stroke as well as significantly less intracerebral hemorrhage in those treated with more aggressive blood pressure control.
Patients with lacunar stroke should be treated with antiplatelet agents.3 Clinicians may select aspirin, clopidogrel, or aspirin with extended-release dipyridamole as first-line strategies for stroke prevention in patients with small vessel disease. Although there are minor differences in efficacy, the specific choice needs to be individualized based on cost, frequency of dosing, and tolerability of side effects. Aspirin is once-daily dosed and typically well-tolerated at doses of 81 or 325 mg. Although aspirin with extended-release dipyridamole has been found to reduce the risk of vascular events compared to aspirin alone in two separate randomized trials,73,74 the modest benefit of the medication may be offset in certain patients due to twice-daily dosing and high rate of headaches as a side effect. Clopidogrel also has been found to be superior to aspirin75 and equivalent to aspirin with extended-release dipyridamole76 for stroke prevention. On the other hand, clopidogrel may interact with commonly prescribed medications (such as proton pump inhibitors), which could potentially increase the risk of cardiovascular events.77 In addition, genetic variants in hepatic metabolism may lead to clopidogrel resistance in certain patients.78 Interestingly, the effects of aspirin and clopidogrel are not additive (like aspirin with extended-release dipyridamole) for stroke prevention, and increase the risks of major bleeding, including intracranial hemorrhage.70,79 Given the potential for harm with long-term therapy, the combination of aspirin and clopidogrel should not be used routinely in chronic stroke prevention if not indicated for other reasons (e.g., cardiac stenting).
Statins also are important in secondary stroke prevention.3 The SPARCL trial randomized patients with stroke or TIA and without known coronary heart disease to atorvastatin 80 mg vs. placebo.80 Patients treated with atorvastatin had significantly lower rates of recurrent stroke and cardiac events, an absolute risk reduction of 2.2% and 3.5%, respectively, despite a small increase in the number of hemorrhagic strokes.
Management of Cardioembolic Stroke. Certain cardioembolic mechanisms of stroke may require short- or long-term anticoagulation or specific surgical treatments. See Table 9 for a list of high-risk cardioembolic stroke mechanisms that may require dedicated anticoagulation as opposed to antiplatelet agents for secondary stroke prevention. Given the possibility for major hemorrhage with anticoagulation, each patient’s individual risk and benefit profile needs to be reviewed prior to initiating this therapy. Certain high-risk conditions, such as mechanical prosthetic valve, require higher targeted INR goals (2.5-3.5),3 and the factor Xa and direct thrombin inhibitors have been shown to be harmful in this population.81 Some patients may require only short-term anticoagulation (e.g., recent anterior wall myocardial infarction or left ventricular thrombus).3 It also should be noted that certain cardioembolic etiologies of stroke pose a particularly high risk for hemorrhagic conversion, and anticoagulation may need to be avoided in these cases. For instance, patients with septic embolization from infective endocarditis have a higher than average risk for intracranial hemorrhage with anticoagulants or fibrinolytics.82 Lastly, some cardioembolic conditions may require specific surgical treatments, such as in the case of the resection of an atrial myxoma.
Table 9. Cardioembolic Stroke Mechanisms that May Warrant Anticoagulation 3 |
|
Mechanical prosthetic valve |
|
Atrial fibrillation |
|
Atrial flutter |
|
Left atrial appendage thrombus |
|
Recent anterior wall myocardial infarction (< 3 months) |
|
Left ventricular thrombus |
|
Dilated cardiomyopathy |
|
Congestive heart failure with EF < 35% and prior stroke |
Management of Atrial Fibrillation. It is well known that anticoagulation reduces the risk of embolic stroke in patients with atrial fibrillation. This reduction in stroke risk has been quantified as 60% with vitamin K antagonists and is superior to antiplatelet therapy.83 Therefore, patients with CHA2DS2-VASc scores of ≥ 2 typically are deemed to benefit from therapeutic anticoagulation, given that this is the cutoff at which the benefit of stroke reduction usually exceeds the average risk of major hemorrhage as an adverse event from anticoagulant usage. (See Table 4.) Scores such as the HAS-BLED score may help quantify risk of intracranial hemorrhage on anticoagulation and identify individuals who may be at higher risk of hemorrhagic complications and thereby should avoid systemic anticoagulants.84 (See Tables 10A and 10B.) Although clinicians commonly withhold anticoagulation in the elderly because of a high risk of falling, this has not been found substantially to lead to excess risk of intracranial hemorrhages. The typical elderly patient would need at least 300 falls per year before the risk of bleeding complications from falling outweighed the benefits of anticoagulation for stroke prevention.85
Table 10A. The HAS-BLED Score 84 |
||
|
H |
Hypertension (SBP > 160 mmHg) |
1 point |
|
A |
Abnormal renal or liver function |
1 point each |
|
S |
Stroke |
1 point |
|
B |
Prior or predisposition to bleeding |
1 point |
|
L |
Labile INRs |
1 point |
|
E |
Elderly (age > 65 years) |
1 point |
|
D |
Drug or alcohol use |
1 point each |
Table 10B. Annual Risk of Major Hemorrhage Stratified by HAS-BLED Score 84 |
|
|
Score |
Rate of Major Hemorrhage |
|
0 |
1.1% |
|
1 |
1.0% |
|
2 |
1.9% |
|
3 |
3.7% |
|
4 |
8.7% |
|
5+ |
12.5% |
More recently, novel oral anticoagulants have been shown to be at least noninferior to warfarin for ischemic stroke prevention and better tolerated in terms of intracranial bleeding risk in patients with nonvalvular atrial fibrillation.86-90 Table 11 summarizes the major trials of the direct thrombin and factor Xa inhibitors. Of these newer anticoagulants, dabigatran at a dose of 150 mg BID and apixaban 5 mg BID were the only agents found to be superior to warfarin for ischemic stroke prevention; however, there was a suggestion of a small increased risk of myocardial infarction with dabigatran when compared to patients treated with warfarin.86 Risk of intracranial hemorrhage was lower with every novel oral anticoagulant than with warfarin. Apart from apixaban,89 all of the newer agents have increased risk of gastrointestinal bleeding events compared with warfarin when given at non-renally adjusted dosages.86,87,90 Additionally, apixaban was the only novel anticoagulant to have a significant benefit over warfarin for all-cause mortality.89 These newer medications all tend to have advantages in their ease of usage, need for monitoring, and drug-drug interactions. Reversal agents now either are approved for use, in the case of idarucizumab for dabigatran reversal,91 or in development for all of the other novel oral anticoagulants (andexanet alfa).92
Table 11. Comparison of Novel Oral Anticoagulants |
|||||
|
Dabigatran |
Rivaroxaban |
Apixaban |
Edoxaban |
||
|
Mechanism |
Thrombin inhibitor |
Xa inhibitor |
Xa inhibitor |
Xa inhibitor |
Xa inhibitor |
|
Trial |
RE-LY |
ROCKET AF |
AVERROES |
ARISTOTLE |
ENGAGE AF-TIMI 48 |
|
Patients |
18,113 |
14,264 |
5,599 |
18,201 |
21,105 |
|
Standard Dosage |
150 mg BID |
20 mg daily |
5 mg BID |
5 mg BID |
60 mg BID |
|
Renal Dosage |
110 mg BID |
15 mg daily |
2.5 mg BID |
2.5 mg BID |
30 mg BID |
|
Comparator |
Warfarin |
Warfarin |
Aspirin |
Warfarin |
Warfarin |
|
Mean CHADS2 |
2.1 |
3.5 |
2.1 |
2.1 |
2.8 |
|
% Prior Stroke/TIA |
20 |
55 |
14 |
19 |
28 |
|
Median TTR, % |
64 |
58 |
N/A |
66 |
68 |
|
Stroke/Systemic Embolism |
1.7% vs. 1.1%* |
2.2% vs .1.7% |
3.7% vs. 1.6% |
1.6% vs. 1.3%* |
1.5% vs. 1.2% |
|
Stroke |
1.6% vs. 1.0% |
NR |
3.4% vs. 1.6% |
1.5% vs. 1.2% |
1.7% vs. 1.5% |
|
Major Bleeding |
3.4% vs. 3.1% |
3.4% vs. 3.5% |
1.2% vs. 1.4% |
3.1% vs. 2.1% |
3.4% vs. 2.8% |
|
GI Bleeding |
1.1% vs. 1.5% |
2.2% vs. 3.2% |
0.4% vs. 0.4% |
0.9% vs. 0.8% |
1.2% vs. 1.5% |
|
Intracranial Hemorrhage |
0.7% vs. 0.3% |
0.7% vs. 0.5% |
0.4% vs. 0.4% |
0.8% vs. 0.3% |
0.9 vs. 0.4% |
|
Mortality |
4.1% vs. 3.6% |
2.2% vs. 1.9% |
4.4% vs. 3.5% |
3.9% vs. 3.5% |
4.4% vs. 4.0% |
|
All outcome results are provided as annual risk, written as rate for warfarin/aspirin arm vs. rate for novel anticoagulant arm (standard dosage); bold denotes significant result. TTR = time in therapeutic range; N/A = not applicable; NR = not reported; * = superiority demonstrated of novel anticoagulant compared with warfarin |
|||||
Management of PFO. Treatment of PFO has been a recently controversial topic. Since PFOs are a common finding, particularly in young stroke patients, the data for their management should be reviewed carefully. Young patients with PFO and stroke should be evaluated for a source of venous thromboembolism or a hypercoagulable state. In the absence of a definite indication for anticoagulation, patients with a PFO often are treated with antiplatelets. There is conflicting evidence as to whether aspirin or anticoagulation is superior, and further high-level data are required to make a decision to treat these patients with anticoagulants over antiplatelets standardly.93,94 There have now been three randomized trials of patients with PFO and stroke evaluating surgical vs. medical management for secondary stroke prevention.36,37,95 None of these trials demonstrated a benefit of percutaneous PFO closure in their intention-to-treat analyses. In one trial, RESPECT, the authors found a benefit of closure in a prespecified analysis of patients who had completed the study per protocol.37 Also, a pooled analysis of these trials showed a modest but significant benefit of closure for the primary composite outcome of stroke, TIA, or death after adjusting for covariates (hazard ratio 0.68; P = 0.049).96 The authors found no significant interaction of closure on the primary outcome based on the size of shunt or the presence of an atrial septal aneurysm. Regardless of surgical treatment, patients with stroke and PFO have relatively low rates of recurrent stroke when treated with medical therapy, which could argue against the routine closure of PFO in stroke patients.
Management of Aortic Arch Atheroma. Similar to atherosclerotic disease elsewhere in the cerebral circulation, warfarin has not been proven to be superior to antiplatelet therapy in patients with aortic arch atheroma. The only randomized trial to directly compare the two antithrombotic regimens, the ARCH trial, randomized patients to dual antiplatelets (low-dose aspirin and clopidogrel 75 mg) or warfarin (INR target range 2-3).97 Although the authors reported a 24% non-significant relative risk reduction with antiplatelet therapy, the study was halted due to low recruitment rates and was underpowered to make any definite clinical claims. The low stroke rates in this trial (e.g., 2.17% per year in the antiplatelet arm) may have been a result of the improved medical management of patients with atherosclerotic cerebrovascular disease in recent years, including aggressive blood pressure control and statin usage. Since there was no antiplatelet monotherapy arm in this trial, it is unclear if single- or dual-agent antiplatelets are preferable in patients with aortic arch atheroma. However, in similar trials employing long-term dual antiplatelets in stroke patients, there has been no increased benefit of additional antiplatelet agents with an increase in major bleeding events.70,79
Management of Large Artery Atherosclerosis. Intracranial atherosclerotic disease is managed medically with antiplatelet agents and aggressive risk factor control, including blood pressure reduction and statin usage.3 The evidence for this recommended practice comes from several major clinical trials comparing antiplatelets to anticoagulants, and medical therapy to intracranial stenting.
Optimal antithrombotic selection was studied in the WASID trial, a randomized trial of warfarin (INR 2-3) vs. aspirin 1,300 mg for angiographically proven 50-99% stenosis of an intracranial vessel.98 The study was stopped early due an increased incidence of bleeding in the warfarin group without any added benefit. Interventional management of intracranial stenosis was studied in the SAMPRISS and VISSIT trials, both of which showed no benefit of intracranial stenting when compared to maximal medical management.41,42 In fact, enrollment in SAMPRISS was halted early since there were significantly higher rates of stroke and death at 30 days in the stenting arm (14.7% vs. 5.8%).41 One-third of the early strokes after stenting were hemorrhages. The results of SAMPRISS were upheld in a recent, similarly designed trial using a different stenting device. The VISSIT trial found that the rate of recurrent stroke or TIA at one year was significantly higher following stenting than if patients were medically managed (34.5% vs. 9.4%), and the majority of these events occurred within the first 30 days.42
In both SAMPRISS and VISSIT, medical therapy proved very effective at reducing recurrent stroke. In SAMPRISS, for example, stroke occurred in 5.8% of patients at 30 days and 12.2% at one year.41 This was lower than expected from previous experience. In both studies, medical treatment was aggressive and included short-term, dual antiplatelet therapy using aspirin 81-325 mg and clopidogrel 75 mg for 90 days. This particular antithrombotic regimen may have been important to the success of the medical management arm in these trials. Dual antiplatelet therapy has been found to reduce microemboli from symptomatic intracranial stenosis,99 and, in the CHANCE trial, short-term dual antiplatelet therapy lowered recurrent stroke rates in patients with high-risk minor stroke and TIA without increasing hemorrhage risk.20 This is being further investigated in the ongoing POINT trial. Medical therapy in the intracranial stenting trials also included aggressive blood pressure control, which was targeted to < 140/90 mmHg or 130/80 mmHg if diabetic.41,42 This contrasts the empiric, non-evidence based practice of prolonged permissive hypertension in these patients in an attempt to augment flow through a known obstruction. Lipid management included an LDL goal of < 70 with the use of high-dose statins, such as 40-80 mg of atorvastatin or 20-40 mg of rosuvastatin.
Extracranial large vessel stenosis is treated with either carotid endarterectomy (CEA) or carotid artery stenting (CAS) for symptomatic stenosis > 50% or asymptomatic stenosis > 70%.46,48 Multiple studies have compared these therapies.47,100 A landmark trial, CREST demonstrated no difference in the outcome of stroke, myocardial infarction, or death between patients (symptomatic and asymptomatic) with moderate to severe stenosis who were treated with CEA vs. stenting. With specific attention to stroke, this outcome occurred more frequently with stenting (4.1% vs. 2.3%), while myocardial infarction occurred more frequently with CEA (2.3% vs. 1.1%). Subgroup analysis from CREST also showed that there was an age difference, with a trend favoring CEA in older patients (older than 70 years of age) and favoring CAS in younger patients.100 Recent 10-year follow-up of CREST continues to show no differences in outcomes between treatment groups, further strengthening the argument for CEA given the association of CAS with up-front stroke risk.101 For any carotid intervention to be beneficial (especially in asymptomatic disease), peri-procedural complications must be < 3%.
Medical therapy for atherosclerosis has improved over the previous 25 years since the initial endarterectomy trials were designed. More aggressive blood pressure control and wider use of statins are now the standard of care. The benefit of CEA or CAS vs. current aggressive medical therapy is not known, and is the focus of the currently enrolling CREST-2 trial. Regardless of whether a clinician pursues conservative or surgical intervention for carotid stenosis, antiplatelet agents and statins should be initiated for secondary prevention of stroke.3 Long-term blood pressure control < 140/90 mmHg should be instituted and hyperglycemia should be avoided. Smoking cessation is important for large artery disease, since tobacco exposure accelerates atherosclerosis.
Controversy still exists regarding the optimal timing of carotid revascularization following an incident TIA or stroke. It was previously believed that a delay of up to 30 days was justified to reduce the risk of peri-procedural complications. However, recent data suggest that revascularization should be performed sooner following stroke, given the high recurrent stroke rates within 14 days and particularly high rates in the first three days.102 Pooled analyses of the major randomized trials of CEA for symptomatic carotid stenosis showed that the time from the vascular event to surgery significantly modifies the benefit of surgery.103 In these trials, the number needed to treat to prevent one ipsilateral stroke in five years was 5 for those randomized within two weeks after an ischemic event vs. 125 for patients randomized after more than 12 weeks.
Management of Stroke of Other Determined Etiology. The dedicated treatment for a stroke mechanism of non-traditional etiology varies and is targeted to the specific etiology. Such treatments range from antimicrobial therapy for infectious stroke etiologies to corticosteroids or other immunosuppressive agents for autoimmune diseases; or from anticoagulation for certain congenital or acquired hypercoagulable states to surgical revascularization in patients with moyamoya disease or fibromuscular dysplasia. Additionally, patients suspected to harbor an occult malignancy should be screened and treated appropriately with surgery, chemotherapy, or radiation as indicated. Patients on estrogen-containing oral contraception or other hormonal therapy should be advised to find an alternative to these medications following a stroke.
Management of Cervical Arterial Dissections. There is no high-level evidence demonstrating that anticoagulation is superior to antiplatelets, or vice versa, for cervical arterial dissection. The only randomized trial studying medical therapy for stroke prevention in dissection patients, the CADISS trial, was a feasibility study and was not powered to be a definitive trial.104 This trial randomized 250 patients with cervical carotid or vertebral dissections to treatment with antiplatelets or anticoagulation. At three months, stroke rates were similar and relatively low in both arms (2% with antiplatelet treatment vs. 1% with anticoagulation). The majority of stroke outcomes occurred early within the follow-up period. The results of this study should be interpreted with caution, due to a lack of adequate power, and, therefore, no expert consensus has been reached on the preferred treatment for dissection. It is important to note that antiplatelets are cheaper, less difficult to manage, and less likely to cause bleeding side effects over long-term follow-up. Some experts advocate for short-term anticoagulation for three to six months, at which time the patient can be converted to antiplatelet therapy if the dissection has healed on repeat neurovascular imaging.
Management of Cancer-related Hypercoagulability. Unfortunately, there is not much high-level evidence on the clinical treatment of cancer patients with stroke, so it is unclear as to what the standard secondary prevention strategy should be in these cases. Therefore, treatment options should take into account the individual prognosis, goals of care, and the wishes of the patient and family who are being treated. A feasibility trial of enoxaparin vs. aspirin therapy in patients with active cancer is currently in enrollment. In the absence of these results, it is generally advised to target antithrombotic selection to the specific stroke mechanism; for instance, antiplatelets would be used for atherosclerotic disease and anticoagulation for cardioembolism. In patients with nonbacterial thrombotic endocarditis (marantic endocarditis), some older data and expert opinion indicate that these patients may benefit particularly from heparinoids over warfarin.105 There are little experience with or published data from the use of novel oral anticoagulants in patients with stroke from cancer-related hypercoagulability.106 Despite medical management, the median survival following stroke in patients with active cancer was found to be only 84 days, with 34% of these patients suffering recurrent thromboembolic events.54
Management of Primary CNS Angiitis. Primary CNS angiitis results in high mortality if left untreated. Due to the rarity of the diagnosis, there are no randomized trials evaluating the management of this form of vasculitis. However, observational evidence has demonstrated that the disease is relatively responsive to corticosteroid therapy, which is the current first-line treatment for primary CNS angiitis.57 Additionally, patients with particularly aggressive disease courses can be treated with cyclophosphamide to achieve remission, and azathioprine or mycophenolate mofetil as steroid-sparing agents for maintenance therapy. More recent cohorts of patients on treatment show that the mortality is 15% at one year and 35% at 10 years, with relapse rates up to 25%.57
Management of Cryptogenic Stroke. It is currently unclear as to whether antiplatelet agents or anticoagulants represent the best medical management for cryptogenic stroke. However, there are multiple currently enrolling trials that are randomizing ESUS patients to either novel oral anticoagulants or antiplatelets. The novel oral anticoagulants were selected to be the comparator in these trials because of their preferential efficacy and safety profile when compared to warfarin.89 Given the high recurrence rate and suspected embolic etiology of cryptogenic strokes, many clinicians advocate for the empiric anticoagulation of patients with ESUS.107 Nevertheless, the AHA/ASA currently does not recommend the standard treatment of cryptogenic stroke patients with anticoagulants.3 The only randomized trial data comparing the efficacy of anticoagulation to antiplatelet therapy in cryptogenic stroke patients are derived from post-hoc analyses of the WARSS trial, which compared the efficacy of adjusted-dose warfarin to aspirin for recurrent ischemic stroke or death within two years. Although the primary analysis of this study showed no benefit of warfarin compared to aspirin, a subgroup analysis of cryptogenic stroke patients with an embolic stroke appearance suggested a nonsignificant trend toward one-third fewer recurrent strokes with warfarin.108 It is unclear if this benefit would be enhanced with the newer anticoagulants. Despite the lack of randomized data, recent emerging evidence linking cardiac abnormalities to cryptogenic strokes have shifted management increasingly in favor of anticoagulation for ESUS patients.62-65
Since the superiority of anticoagulation is not established in cryptogenic stroke patients, it is reasonable to aggressively search to uncover occult embolic mechanisms that would support the evidenced-based use of anticoagulation for secondary stroke prevention. Atrial fibrillation patients with prior stroke or TIA score at least 2 points on the CHA2DS2-VASc scale, which would indicate that these patients would likely benefit from anticoagulation.35 Therefore, if patients with embolic-appearing cryptogenic strokes are discovered to have atrial fibrillation on hospital follow-up, anticoagulation most likely would be warranted. Recent studies have proven that prolonged arrhythmia monitoring can potentially lead to large-scale changes in management within certain stroke populations.66,67 In the EMBRACE study, the use of an extended cardiac event recorder in cryptogenic stroke patients significantly increased the rate of atrial fibrillation detection compared to standard monitoring at 30 days (16.1% vs. 3.2).66 Similarly, the CRYSTAL-AF trial demonstrated that the use of an implantable loop recorder increases the yield of atrial fibrillation detection (8.9% vs. 1.4% at six months, 12.4% vs. 2.0% at one year, and 30% vs. 3.0% at three years).67 The median time from randomization to arrhythmia detection in the CRYSTAL-AF study was 84 days. The bottom line is that if covert atrial fibrillation is suspected as an etiology of cryptogenic stroke, then prolonged cardiac monitoring is recommended, with a higher yield the longer the monitoring lasts. In the CRYSTAL-AF study, 97% of patients in whom atrial fibrillation had been demonstrated were treated with anticoagulation at 12 months, implying a change in management based on the clinical detection of atrial fibrillation. It is still unclear as to how increased atrial fibrillation detection with longer monitoring affects stroke outcomes, and whether the burden of atrial fibrillation modifies this effect size. However, given the increased rate of anticoagulant usage in the CRYSTAL-AF trial as a result of increased atrial fibrillation detection,67 it is inferred that there may be a theoretical benefit of prolonged monitoring on cardioembolic stroke recurrence due to the known superiority of anticoagulation to antiplatelets in medium- to high-risk atrial fibrillation patients. However, this has yet to be proven definitively. In the meantime, a standard approach is to monitor cryptogenic stroke patients for at least 30 days, while treating them with antiplatelet therapy, unless atrial fibrillation is discovered.107
In addition to extended arrhythmia monitoring, it has been suggested that additional advanced diagnostic techniques be used to uncover other potential stroke etiologies in cryptogenic patients who have otherwise unremarkable routine stroke evaluations.60 Such testing could include the measurement of D-dimer and screening for occult malignancy to rule out cancer-related coagulopathy. Aortic pathology such as atherosclerosis also may embolize to the brain and is best visualized using transesophageal echocardiography. Interatrial shunts, including PFO and atrial septal defects, should be tested for with bubble studies either using echocardiography or transcranial Doppler. Advanced, high-resolution vascular wall imaging, with or without contrast, also may detect arterial plaques with ulceration or other high-risk elements that could serve as a source of embolism.
Conclusion
Given the frequency of stroke occurrence, and the high rates of resultant death and disability, an evidence-based prevention strategy is one of the largest weapons in a clinican’s armamentarium against stroke. With time, we are learning more about the importance of precise risk factor management, the role of statins for atherosclerotic disease, and the use of targeted antithrombotic therapy based on the underlying mechanism of stroke. Whereas large artery atherosclerotic and small vessel disease responds to antiplatelet agents, anticoagulation has been found to be superior in certain high-risk cardiac conditions, such as atrial fibrillation. The recent expansion of oral anticoagulants to include factor Xa and direct thrombin inhibitors has significantly improved the overall risk/benefit profile of the medical therapy for patients with nonvalvular atrial fibrillation. Surgical options also vary by stroke subtype and mechanism. Although symptomatic cervical carotid stenosis is amenable to revascularization with stenting or endarterectomy, stenting of intracranial atherosclerosis has proven harmful in clinical trials. Patients with unexplained stroke after a standard diagnostic evaluation, particularly if young, should have advanced testing to evaluate for rarer causes of stroke. If a cryptogenic stroke appears embolic on neuroimaging, such patients may benefit from extended cardiac monitoring or empiric anticoagulation, although randomized trial data are needed to demonstrate improved clinical outcomes with such practice.
REFERENCES
- Mozaffarian D, Benjamin EJ, Go AS, et al. Heart Disease and Stroke Statistics—2015 Update: a report from the American Heart Association. Circulation 2015;131:e29-322.
- Mozaffarian D, Benjamin EJ, Go AS, et al. Heart Disease and Stroke Statistics—2016 Update: A Report From the American Heart Association. Circulation 2016;133:e38-e360.
- Kernan WN, Ovbiagele B, Black HR, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2014;45:2160-2236.
- Wright JT, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015;373:2103-2116.
- Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;129(25 Suppl 2):S1-45.
- Group HPSC. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: A randomised placebo-controlled trial. Lancet 2002;360:7-22.
- Yaggi HK, Concato J, Kernan WN, et al. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med 2005;353:2034-2041.
- Gami AS, Pressman G, Caples SM, et al. Association of atrial fibrillation and obstructive sleep apnea. Circulation 2004;110:364-367.
- Barrett-Connor E, Khaw KT. Diabetes mellitus: an independent risk factor for stroke? Am J Epidemiol 1988;128:116-123.
- Kleindorfer D, Panagos P, Pancioli A, et al. Incidence and short-term prognosis of transient ischemic attack in a population-based study. Stroke 2005;36:720-723.
- Redgrave JN, Coutts SB, Schulz UG, et al. Systematic review of associations between the presence of acute ischemic lesions on diffusion-weighted imaging and clinical predictors of early stroke risk after transient ischemic attack. Stroke 2007;38:1482-1488.
- Easton JD, Saver JL, Albers GW, et al. Definition and evaluation of transient ischemic attack: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; and the Interdisciplinary Council on Peripheral Vascular Disease. The American Academy of Neurology affirms the value of this statement as an educational tool for neurologists. Stroke 2009;40:2276-2293.
- Prabhakaran S, Chong JY, Sacco RL. Impact of abnormal diffusion-weighted imaging results on short-term outcome following transient ischemic attack. Arch Neurol 2007;64:1105-1109.
- Ovbiagele B, Kidwell CS, Saver JL. Epidemiological impact in the United States of a tissue-based definition of transient ischemic attack. Stroke 2003;34:919-924.
- Johnston SC, Rothwell PM, Nguyen-Huynh MN, et al. Validation and refinement of scores to predict very early stroke risk after transient ischaemic attack. Lancet 2007;369:283-292.
- Amarenco P, Lavallée PC, Labreuche J, et al. One-year risk of stroke after transient ischemic attack or minor stroke. N Engl J Med 2016;374:1533-1542.
- Olivot JM, Wolford C, Castle J, et al. Two aces: transient ischemic attack work-up as outpatient assessment of clinical evaluation and safety. Stroke 2011;42:1839-1843.
- Wardlaw JM, Brazzelli M, Chappell FM, et al. ABCD2 score and secondary stroke prevention: Meta-analysis and effect per 1,000 patients triaged. Neurology 2015;85:373-380.
- Rothwell PM, Giles MF, Chandratheva A, et al. Effect of urgent treatment of transient ischaemic attack and minor stroke on early recurrent stroke (EXPRESS study): A prospective population-based sequential comparison. Lancet 2007;370:1432-1442.
- Wang Y, Zhao X, Liu L, et al. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. N Engl J Med 2013;369:11-19.
- Libman RB, Wirkowski E, Alvir J, et al. Conditions that mimic stroke in the emergency department. Implications for acute stroke trials. Arch Neurol 1995;52:1119-1122.
- Chernyshev OY, Martin-Schild S, Albright KC, et al. Safety of tPA in stroke mimics and neuroimaging-negative cerebral ischemia. Neurology 2010;74:1340-1345.
- Amort M, Fluri F, Schäfer J, et al. Transient ischemic attack versus transient ischemic attack mimics: Frequency, clinical characteristics and outcome. Cerebrovasc Dis 2011;32:57-64.
- Josephson SA, Sidney S, Pham TN, et al. Higher ABCD2 score predicts patients most likely to have true transient ischemic attack. Stroke 2008;39:3096-3098.
- Zinkstok SM, Engelter ST, Gensicke H, et al. Safety of thrombolysis in stroke mimics: Results from a multicenter cohort study. Stroke 2013;44:1080-1084.
- Mohr J, Wolf PA, Grotta JC, et al. Stroke: Pathophysiology, Diagnosis, and Management. 5th ed. Philadelphia, PA: Elsevier Saunders; 2011.
- Adams HP, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993;24:35-41.
- Bogousslavsky J, Cachin C, Regli F, et al. Cardiac sources of embolism and cerebral infarction—clinical consequences and vascular concomitants: The Lausanne Stroke Registry. Neurology 1991;41:855-859.
- Rascol A, Clanet M, Manelfe C, et al. Pure motor hemiplegia: CT study of 30 cases. Stroke 1982;13:11-17.
- Fisher CM. Lacunes: Small, deep cerebral infarcts. Neurology 1965;15:774-784.
- Fisher CM. Lacunar strokes and infarcts: A review. Neurology 1982;32:871-876.
- McArdle PF, Kittner SJ, Ay H, et al. Agreement between TOAST and CCS ischemic stroke classification: The NINDS SiGN study. Neurology 2014;83:1653-1660.
- Moulin T, Tatu L, Vuillier F, et al. Role of a stroke data bank in evaluating cerebral infarction subtypes: Patterns and outcome of 1,776 consecutive patients from the Besançon stroke registry. Cerebrovasc Dis 2000;10:261-271.
- Fieschi C, Argentino C, Lenzi GL, et al. Clinical and instrumental evaluation of patients with ischemic stroke within the first six hours. J Neurol Sci 1989;91:311-321.
- Camm AJ, Kirchhof P, Lip GY, et al. Guidelines for the management of atrial fibrillation: The Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur Heart J 2010;31:2369-2429.
- Furlan AJ, Reisman M, Massaro J, et al. Closure or medical therapy for cryptogenic stroke with patent foramen ovale. N Engl J Med 2012;366:991-999.
- Carroll JD, Saver JL, Thaler DE, et al. Closure of patent foramen ovale versus medical therapy after cryptogenic stroke. N Engl J Med 2013;368:1092-1100.
- Tunick PA, Nayar AC, Goodkin GM, et al. Effect of treatment on the incidence of stroke and other emboli in 519 patients with severe thoracic aortic plaque. Am J Cardiol 2002;90:1320-1325.
- Vaduganathan P, Ewton A, Nagueh SF, et al. Pathologic correlates of aortic plaques, thrombi and mobile “aortic debris” imaged in vivo with transesophageal echocardiography. J Am Coll Cardiol 1997;30:357-363.
- Atherosclerotic disease of the aortic arch as a risk factor for recurrent ischemic stroke. The French Study of Aortic Plaques in Stroke Group. N Engl J Med 1996;334:1216-1221.
- Chimowitz MI, Lynn MJ, Derdeyn CP, et al. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med 2011;365:993-1003.
- Zaidat OO, Fitzsimmons BF, Woodward BK, et al. Effect of a balloon-expandable intracranial stent vs medical therapy on risk of stroke in patients with symptomatic intracranial stenosis: The VISSIT randomized clinical trial. JAMA 2015;313:1240-1248.
- Kasner SE, Chimowitz MI, Lynn MJ, et al. Predictors of ischemic stroke in the territory of a symptomatic intracranial arterial stenosis. Circulation 2006;113:555-563.
- Nishimaru K, McHenry LC, Toole JF. Cerebral angiographic and clinical differences in carotid system transient ischemic attacks between American Caucasian and Japanese patients. Stroke 1984;15:56-59.
- Hass WK, Fields WS, North RR, et al. Joint study of extracranial arterial occlusion. II. Arteriography, techniques, sites, and complications. JAMA 1968;203:961-968.
- Barnett HJ, Taylor DW, Eliasziw M, et al. Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med 1998;339:1415-1425.
- Rosenfield K, Matsumura JS, Chaturvedi S, et al. Randomized trial of stent versus surgery for asymptomatic carotid stenosis. N Engl J Med 2016;374:1011-1020.
- Halliday A, Harrison M, Hayter E, et al. 10-year stroke prevention after successful carotid endarterectomy for asymptomatic stenosis (ACST-1): A multicentre randomised trial. Lancet 2010;376:1074-1084.
- Ferro JM, Massaro AR, Mas JL. Aetiological diagnosis of ischaemic stroke in young adults. Lancet Neurol 2010;9:1085-1096.
- Debette S, Leys D. Cervical-artery dissections: predisposing factors, diagnosis, and outcome. Lancet Neurol 2009;8:668-678.
- Patel RR, Adam R, Maldjian C, et al. Cervical carotid artery dissection: Current review of diagnosis and treatment. Cardiol Rev 2012;20:145-152.
- Cestari DM, Weine DM, Panageas KS, et al. Stroke in patients with cancer: Incidence and etiology. Neurology 2004;62:2025-2030.
- Nguyen T, DeAngelis LM. Stroke in cancer patients. Curr Neurol Neurosci Rep 2006;6:187-192.
- Navi BB, Singer S, Merkler AE, et al. Recurrent thromboembolic events after ischemic stroke in patients with cancer. Neurology 2014;83:26-33.
- Navi BB, Singer S, Merkler AE, et al. Cryptogenic subtype predicts reduced survival among cancer patients with ischemic stroke. Stroke 2014;45:2292-2297.
- Kim SJ, Park JH, Lee MJ, et al. Clues to occult cancer in patients with ischemic stroke. PLoS One 2012;7:e44959.
- Salvarani C, Brown RD, Christianson T, et al. An update of the Mayo Clinic cohort of patients with adult primary central nervous system vasculitis: Description of 163 patients. Medicine (Baltimore) 2015;94:e738.
- Miller DV, Salvarani C, Hunder GG, et al. Biopsy findings in primary angiitis of the central nervous system. Am J Surg Pathol 2009;33:35-43.
- Powers WJ. Primary angiitis of the central nervous system: Diagnostic criteria. Neurol Clin 2015;33:515-526.
- Bang OY, Ovbiagele B, Kim JS. Evaluation of cryptogenic stroke with advanced diagnostic techniques. Stroke 2014;45:1186-1194.
- Hart RG, Diener HC, Coutts SB, et al. Embolic strokes of undetermined source: the case for a new clinical construct. Lancet Neurol 2014;13:429-438.
- Kamel H, Elkind MS, Bhave PD, et al. Paroxysmal supraventricular tachycardia and the risk of ischemic stroke. Stroke 2013;44:1550-1554.
- Llombart V, Antolin-Fontes A, Bustamante A, et al. B-type natriuretic peptides help in cardioembolic stroke diagnosis: Pooled data meta-analysis. Stroke 2015;46:1187-1195.
- Kamel H, Soliman EZ, Heckbert SR, et al. P-wave morphology and the risk of incident ischemic stroke in the Multi-Ethnic Study of Atherosclerosis. Stroke 2014;45:2786-2788.
- Yaghi S, Moon YP, Mora-McLaughlin C, et al. Left atrial enlargement and stroke recurrence: The Northern Manhattan Stroke Study. Stroke 2015;46:1488-1493.
- Gladstone DJ, Spring M, Dorian P, et al. Atrial fibrillation in patients with cryptogenic stroke. N Engl J Med 2014;370:2467-2477.
- Sanna T, Diener HC, Passman RS, et al. Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med 2014;370:2478-2486.
- Gupta A, Gialdini G, Giambrone AE, et al. Association between nonstenosing carotid artery plaque on MR angiography and acute ischemic stroke. JACC Cardiovasc Imaging 2016: In press.
- Gupta A, Gialdini G, Lerario MP, et al. Magnetic resonance angiography detection of abnormal carotid artery plaque in patients with cryptogenic stroke. J Am Heart Assoc 2015;4:e002012.
- Benavente OR, Hart RG, McClure LA, et al. Effects of clopidogrel added to aspirin in patients with recent lacunar stroke. N Engl J Med 2012;367:817-825.
- Ntaios G, Papavasileiou V, Milionis H, et al. Embolic strokes of undetermined source in the Athens Stroke Registry: An outcome analysis. Stroke 2015;46:2087-2093.
- Benavente OR, Coffey CS, Conwit R, et al. Blood-pressure targets in patients with recent lacunar stroke: The SPS3 randomised trial. Lancet 2013;382:507-515.
- Diener HC, Cunha L, Forbes C, et al. European Stroke Prevention Study. 2. Dipyridamole and acetylsalicylic acid in the secondary prevention of stroke. J Neurol Sci 1996;143:1-13.
- Halkes PH, van Gijn J, Kappelle LJ, et al. Aspirin plus dipyridamole versus aspirin alone after cerebral ischaemia of arterial origin (ESPRIT): Randomised controlled trial. Lancet 2006;367:1665-1673.
- Committee CS. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). CAPRIE Steering Committee. Lancet 1996;348:1329-1339.
- Sacco RL, Diener HC, Yusuf S, et al. Aspirin and extended-release dipyridamole versus clopidogrel for recurrent stroke. N Engl J Med 2008;359:1238-1251.
- Sherwood MW, Melloni C, Jones WS, et al. Individual proton pump inhibitors and outcomes in patients with coronary artery disease on dual antiplatelet therapy: A systematic review. J Am Heart Assoc 2015;4: pii: e002245.
- Hulot JS, Bura A, Villard E, et al. Cytochrome P450 2C19 loss-of-function polymorphism is a major determinant of clopidogrel responsiveness in healthy subjects. Blood 2006;108:2244-2247.
- Diener HC, Bogousslavsky J, Brass LM, et al. Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high-risk patients (MATCH): Randomised, double-blind, placebo-controlled trial. Lancet 2004;364:331-337.
- Amarenco P, Bogousslavsky J, Callahan A, et al. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med 2006;355:549-559.
- Eikelboom JW, Connolly SJ, Brueckmann M, et al. Dabigatran versus warfarin in patients with mechanical heart valves. N Engl J Med 2013;369:1206-1214.
- Asaithambi G, Adil MM, Qureshi AI. Thrombolysis for ischemic stroke associated with infective endocarditis: Results from the nationwide inpatient sample. Stroke 2013;44:2917-2919.
- Hart RG, Pearce LA, Aguilar MI. Meta-analysis: Antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med 2007;146:857-867.
- Pisters R, Lane DA, Nieuwlaat R, et al. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: The Euro Heart Survey. Chest 2010;138:1093-1100.
- Sellers MB, Newby LK. Atrial fibrillation, anticoagulation, fall risk, and outcomes in elderly patients. Am Heart J 2011;161:241-246.
- Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009;361:1139-1151.
- Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011;365:883-891.
- Connolly SJ, Eikelboom J, Joyner C, et al. Apixaban in patients with atrial fibrillation. N Engl J Med 2011;364:806-817.
- Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011;365:981-992.
- Giugliano RP, Ruff CT, Braunwald E, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2013;369:2093-2104.
- Pollack CV, Reilly PA, Eikelboom J, et al. Idarucizumab for dabigatran reversal. N Engl J Med 2015;373:511-520.
- Siegal DM, Curnutte JT, Connolly SJ, et al. Andexanet alfa for the reversal of factor Xa inhibitor activity. N Engl J Med 2015;373:2413-2424.
- Kitsios GD, Dahabreh IJ, Abu Dabrh AM, et al. Patent foramen ovale closure and medical treatments for secondary stroke prevention: A systematic review of observational and randomized evidence. Stroke 2012;43:422-431.
- Homma S, Sacco RL, Di Tullio MR, et al; Investigators PiCSSP. Effect of medical treatment in stroke patients with patent foramen ovale: patent foramen ovale in Cryptogenic Stroke Study. Circulation 2002;105:2625-2631.
- Meier B, Kalesan B, Mattle HP, et al. Percutaneous closure of patent foramen ovale in cryptogenic embolism. N Engl J Med 2013;368:1083-1091.
- Kent DM, Dahabreh IJ, Ruthazer R, et al. Device closure of patent foramen ovale after stroke: Pooled analysis of completed randomized trials. J Am Coll Cardiol 2016;67:907-917.
- Amarenco P, Davis S, Jones EF, et al. Clopidogrel plus aspirin versus warfarin in patients with stroke and aortic arch plaques. Stroke 2014;45:1248-1257.
- Chimowitz MI, Lynn MJ, Howlett-Smith H, et al. Comparison of warfarin and aspirin for symptomatic intracranial arterial stenosis. N Engl J Med 2005;352:1305-1316.
- Wong KS, Chen C, Fu J, et al. Clopidogrel plus aspirin versus aspirin alone for reducing embolisation in patients with acute symptomatic cerebral or carotid artery stenosis (CLAIR study): A randomised, open-label, blinded-endpoint trial. Lancet Neurol 2010;9:489-497.
- Brott TG, Hobson RW, Howard G, et al. Stenting versus endarterectomy for treatment of carotid-artery stenosis. N Engl J Med 2010;363:11-23.
- Brott TG, Howard G, Roubin GS, et al. Long-term results of stenting versus endarterectomy for carotid-artery stenosis. N Engl J Med 2016;374:1021-1031.
- Johansson E, Cuadrado-Godia E, Hayden D, et al. Recurrent stroke in symptomatic carotid stenosis awaiting revascularization: A pooled analysis. Neurology 2016;86:498-504.
- Rothwell PM, Eliasziw M, Gutnikov SA, et al; Collaboration CET. Endarterectomy for symptomatic carotid stenosis in relation to clinical subgroups and timing of surgery. Lancet 2004;363:915-924.
- Markus HS, Hayter E, Levi C, et al. Antiplatelet treatment compared with anticoagulation treatment for cervical artery dissection (CADISS): A randomised trial. Lancet Neurol 2015;14:361-367.
- Rogers LR, Cho ES, Kempin S, Posner JB. Cerebral infarction from non-bacterial thrombotic endocarditis. Clinical and pathological study including the effects of anticoagulation. Am J Med 1987;83:746-756.
- Yaghi S, Kamel H, Elkind MS. Potential new uses of non-vitamin K antagonist oral anticoagulants to treat and prevent stroke. Neurology 2015;85:1078-1084.
- Zhang C, Kasner S. Diagnosis, prognosis, and management of cryptogenic stroke. F1000Res 2016;5 doi: 10.12688/f1000research.7384.1. eCollection 2016.
- Sacco RL, Prabhakaran S, Thompson JL, et al. Comparison of warfarin versus aspirin for the prevention of recurrent stroke or death: Subgroup analyses from the Warfarin-Aspirin Recurrent Stroke Study. Cerebrovasc Dis 2006;22:4-12.
MONOGRAPH: Here is the best evidence-based practice for stroke diagnosis, prevention, and risk factor management.
Subscribe Now for Access
You have reached your article limit for the month. We hope you found our articles both enjoyable and insightful. For information on new subscriptions, product trials, alternative billing arrangements or group and site discounts please call 800-688-2421. We look forward to having you as a long-term member of the Relias Media community.

 B
B 
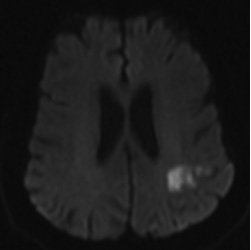 B
B 
 D
D 
 B
B 