Vaginal Bleeding in the Nonpregnant Patient
Vaginal Bleeding in the Nonpregnant Patient
Author:
Pam Arsove, MD, FACEP, Associate Residency Director, Department of Emergency Medicine, Long Island Jewish Medical Center, New Hyde Park, NY; Assistant Professor of Emergency Medicine, Hofstra North Shore Long Island Jewish School of Medicine.
Peer Reviewer:
Jonathan Glauser, MD, FACEP, Faculty, MetroHealth Residency Emergency Medicine, Associate Professor of Emergency Medicine, Case Western Reserve University, Cleveland, OH.
Introduction
Abnormal uterine bleeding affects females of all ages and is a common complaint in gynecologic practice. An estimated $1 billion annually is spent on direct costs for this diagnosis, with another $12 billion attributed to indirect costs such as health-related supplies and missed employment.1 In addition, women with abnormal uterine bleeding report a lower score on health-related questionnaires that deal with quality of life.2 They frequently seek care in the emergency department (ED) to determine if they are pregnant or if they have a concern about the amount or duration of bleeding.
All women of childbearing age who present to the ED with vaginal bleeding should have a pregnancy test to exclude pregnancy-related complications. If pregnancy is suspected by history and the urine human chorionic gonadotropin (hCG) is negative, a serum beta-hCG should be performed as the definitive test in the rare event of a falsely negative urine hCG. While the definitive diagnosis of non-pregnancy-related vaginal bleeding often is made after the patient leaves the ED, the physician must have a solid understanding of the etiology and pathophysiology of various causes of bleeding to provide appropriate ED care, counseling, and disposition. This article will review the common causes of vaginal bleeding in the nonpregnant patient. Pre-pubertal bleeding will not be reviewed in this article. Table 1 lists the common causes of bleeding in the pre- and postmenopausal patient. It is common practice to focus on dysfunctional uterine bleeding (DUB) as the cause of bleeding in the premenopausal woman, and malignancy in the postmenopausal woman. While these are common concerns, the ED physician should not overlook the other diagnostic possibilities.
Table 1: Common Causes of Vaginal Bleeding in the Pre- and Postmenopausal Female
|
Premenopausal |
Postmenopausal |
|
|
Pathophysiology
Although normal variations occur, it is standard practice to consider a normal menstrual cycle as beginning on the first day of bleeding, occurring every 28 days (± 7 days), and lasting approximately 4 days. Hormonal factors that regulate menstruation include estrogen, progesterone, follicle-stimulating hormone (FSH), and luteinizing hormone (LH). Figure 1 illustrates the effect these hormones have on the endometrium and ovaries in a typical menstrual cycle. Once menses is finished, the follicular phase of the menstrual cycle begins. The developing ovary secretes estrogen, which causes the endometrial lining to thicken and the pituitary gland to release FSH and LH, leading to ovulation. The resulting corpus luteum (CL) produces progesterone, which maintains the endometrial lining in preparation for implantation in the luteal phase of the menstrual cycle. In the nonpregnant patient, the CL involutes, and estrogen and progesterone drop quickly. This sudden decrease in estrogen and progesterone causes sloughing of the endometrial lining, and menses occurs, starting the cycle all over again. Any physiologic process that alters this normal cycle may result in abnormal uterine bleeding.
Figure 1: Normal Menstrual Cycle
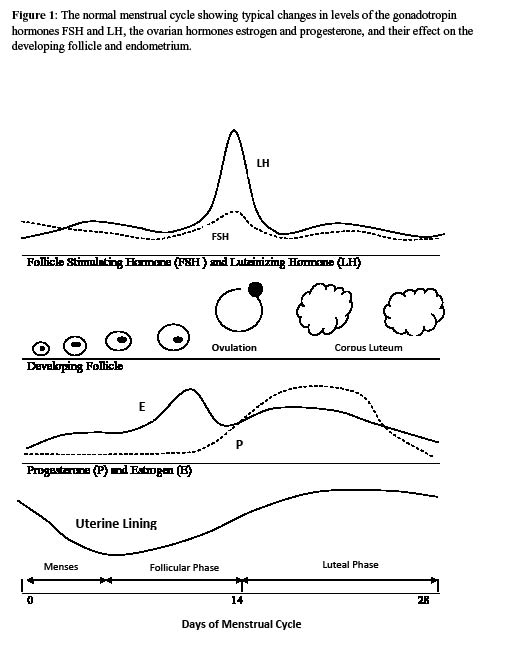
The normal menstrual cycle, showing typical changes in levels of the gonadotropin hormones FSH and LH, the ovarian hormones estrogen and progesterone, and their effect on the developing follicle and endometrium.
General Considerations for Managing Vaginal Bleeding in the ED
Hemodynamic stability is the most important initial concern for patients with vaginal bleeding. The hemodynamically unstable patient who is actively bleeding is resuscitated with fluid, packed red blood cells, and vasopressors, if indicated. An emergent pelvic examination is performed to evaluate for a visible cause of bleeding, such as foreign objects, trauma, or tumors. Patients with no obvious cause and continual instability may be started on a trial of intravenous Premarin 25 mg given slowly, a conjugated estrogen that decreases uterine blood flow. If instability persists, the patient must be prepared for emergent surgery.
The ED work-up of a stable patient depends on the history and physical exam, which should include orthostatic vital signs and a pelvic exam. Only laboratory and ancillary testing that will impact the ED management and disposition of the patient is performed.
Premenopausal Vaginal Bleeding
Premenopausal patients presenting to the ED usually complain of excessive, prolonged, or irregular bleeding. It is not uncommon for physicians to confuse terminology.3 Menorrhagia refers to prolonged or excessive bleeding that follows a patient's normal menstrual cycle. It is generally referred to as menses lasting longer than a week or with more than 80 cc of blood loss. Metrorrhagia refers to intermenstrual bleeding. When women complain of both patterns of irregular bleeding, it is termed menometrorrhagia. Hypomenorrhea refers to a decreased flow or shortened menses, while oligomenorrhea refers to menstrual cycles longer than 35 days, and polymenorrhea denotes bleeding at intervals less than 21 days. When a patient is on hormone replacement, the term breakthrough bleeding is used to describe metrorrhagia associated with their use, while withdrawal bleeding describes the bleeding that follows abrupt cessation of progestin. Dysfunctional uterine bleeding (DUB) refers to abnormal uterine bleeding when no pathology or systemic disease is found.
In a normal menstrual cycle, approximately 40 cc of blood loss occurs. Be aware that a patient's subjective assessment of the amount of blood loss, duration of bleeding, and amount of sanitary products used are poor correlates with measured blood loss.4 When a patient describes abnormal bleeding, the important objective information for the ED physician will be gained by a thorough physical exam, including a pelvic exam, and laboratory evaluation when indicated.
Dysfunctional Uterine Bleeding
When organic disease has been excluded as a cause of a patient's bleeding, it is termed DUB. While the ED physician usually encounters patients in whom the diagnosis is unknown, most premenopausal women with a normal physical exam will have DUB. DUB is usually caused by a hormonal problem, and is divided into two main categories: anovulatory and ovulatory. Anovulatory and ovulatory bleeding account for approximately 90% and 10%, respectively, of all cases of DUB.5
Anovulatory bleeding is the most common cause of DUB in the premenopausal female, especially at the extremes of reproductive ages. Most cases occur in adolescents whose hypothalamic pituitary axis is not fully mature,6 and premenopausal women who have abnormal cyclic hormone production causing low estrogen levels.7,8 It is estimated to be the cause of dysfunctional bleeding in 95% of females younger than 20 years of age, 20% of females between the ages of 20 and 40, and 90% of women who are within several years of menopause.9 Anovulatory bleeding is characterized by lack of ovulation, absent luteal phase of the menstrual cycle, and, subsequently, no progesterone secretion by the ovaries. The result is unopposed estrogen stimulation, which produces erratic and excessive endometrial growth and irregular bleeding. Anovulatory cycles have unpredictable bleeding patterns that may produce a range of bleeding abnormalities, including significant hemorrhage. The most common bleeding pattern is periods of amenorrhea with episodic menorrhagia. Menometrorrhagia and polymenorrhea are common. Anovulatory bleeding may be caused by a number of non-gynecological conditions that cause hormonal changes affecting ovulation, such as polycystic ovarian syndrome (POC) and endocrine disorders. Table 2 lists common predisposing factors for DUB. The most common complications from anovulatory DUB are anemia, and infertility in reproductive-age females.
Table 2: Anovulatory Causes of Vaginal Bleeding in the Premenopausal Patient
Anovulatory
Unopposed estrogen secretion:
- Postmenarche and perimenopause
- Polycystic ovarian syndrome
- Extreme obesity
- Oral contraceptive use
- Estrogen replacement therapy
- Stress and illness
- Thyroid disorders
- Hyperprolactinemia
- Idiopathic
Ovulatory bleeding is seen in a minority of patients with DUB. It is characterized by menorrhagia or intermenstrual bleeding that accompanies regular menstrual cycles. Ovulatory bleeding is less well characterized, but is attributed to hormonal abnormalities most commonly seen in adolescence and perimenopausal women. Recent literature describes a "LOOP" (luteal out of phase) phenomenon to explain ovulatory abnormal bleeding in perimenopausal women that involves abnormal high estradiol levels persisting from the mid-luteal cycle into the menstrual and follicular phases of the next menstrual cycle.10
The goal in treating DUB is to reduce bleeding. The initial approach to the patient depends on whether she presents with an acute bleeding episode or with chronic abnormal bleeding.
Medical management is the initial treatment of choice in stable patients with chronic bleeding who want to maintain fertility and those close to menopause. The medications used to decrease bleeding work by normalizing the menstrual cycle, reducing bleeding, or stopping menstruation altogether. (See Table 3.) Because most treatment is ongoing and requires an exclusion of organic disease, it is usually prescribed by the patient's gynecologist. The work-up to exclude organic disease is rarely indicated in the ED. Patients with diagnosed DUB often require chronic therapy after the initial episode of bleeding resolves. The common medications used in DUB are listed below, and comparisons of the mechanisms of action, advantages, and disadvantages are listed in Tables 4A and 4B.
Table 3: A Comparison of the Effects of Medication Use in Dysfunctional Uterine Bleeding
|
Medication |
Normalizes Menses |
Reduces Bleeding |
Stops Menses |
|
Progestins |
+ |
+ |
|
|
Estrogen |
+ |
||
|
OCPs |
+ |
+ |
|
|
NSAIDs |
+ |
||
|
Tranexamic acid |
+ |
||
|
GnRH antagonists |
+ |
||
|
Androgens |
+ |
± |
Hormonal Therapies. Progestins are considered by many physicians to be the first-line treatment for DUB. They are synthetic progesterones that stop endometrial proliferation and are commonly used for anovulatory bleeding because they replace the progesterone deficiency in this disorder and restore normal cyclic menstrual periods. Progestins may be given in short cyclical intervals. Medroxyprogesterone acetate 10 mg orally for 10 days on day 12-25 of the menstrual cycle is commonly used. In perimenopausal females, estrogen therapy is often given prior to progestins to replace their estrogen-deprived endometrium.
Estrogens are very effective in controlling significant acute bleeding. They cause vasospasm on a capillary level through an effect on several coagulation factors, platelets, and capillary permeability. High-dose estrogen therapy may be administered for up to 48 hours to the acutely hemorrhaging patient. Conjugated estrogens are also given orally to perimenopausal women who are estrogen-deficient and experience DUB. In these cases, progestins are given concurrently.
Table 4A: Medication to Treat Dysfunctional Uterine Bleeding: Hormonal
|
Medication |
Mechanism of Action |
Advantage |
Disadvantage |
|
OCPs |
Decreases GnRH, FSH, and LH, prevents ovulation, decreases endometrial growth |
Provides long-term management and contraception, regulates the menstrual cycle |
Often recurs when therapy is stopped, withdrawal bleeding occurs |
|
Progestin |
Inhibits endometrial cell proliferation |
May be administered in IUD form, regulates the menstrual cycle |
Not effective in acute bleeding, select patients may require pretreatment estrogen therapy |
|
Estrogen |
Rapid regrowth of endometrium, produces platelet plug, causes capillary vasospasm |
Effective in profuse, acute bleeding |
May require up to 48 hours of treatment to determine efficacy |
|
GnRH agonists |
Suppresses gonadotropin release, causing hypogonadism |
Produces amenorrhea, thins endometrium prior to surgery |
Menopausal side effects, osteoporosis, expensive |
|
Male hormones |
Suppresses gonadotropin release, causing hypogonadism |
Thins endometrium prior to surgery, does not cause osteoporosis seen with GnRH agonists |
Irreversible masculinization, expensive |
Table 4B: Medication to Treat Dysfunctional Uterine Bleeding: Non-hormonal
|
Medication |
Mechanism of Action |
Advantage |
Disadvantage |
|
NSAIDs |
Inhibits prostaglandin synthesis, causing vasoconstriction and increased platelet aggregation |
Inexpensive, effective in ovulatory dysfunctional uterine bleeding, pain relief, safe for long-term use, can be used in combination with hormonal therapy |
Limited efficacy in anovulatory dysfunctional uterine bleeding |
|
Tranexamic acid |
Inhibits degradation of fibrin within endometrial vessels |
Very effective in reducing bleeding |
Expensive, safety and efficacy unknown in postmenopausal patients and those < 18 years, questionable risk of thromboembolic disease |
Oral contraceptive pills (OCPs) suppress epithelial development and restore normal cyclic menstruation. OCPs containing both estrogen and progestins are often used in the long-term management of DUB. They are usually effective within 24 hours and may be given daily in mild cases. Higher doses for shorter intervals may be given if bleeding is more significant.
Gonadotropin-releasing hormone (GnRH) agonists and androgenic male hormones such as danazol are often a last-resort treatment option for DUB because of their high side-effect profile. GnRH agonists induce a medical menopause and are considered for women in whom all other treatment options have failed who still desire pregnancy. Androgens cause unpleasant and often irreversible masculinization. Both may be given to patients prior to surgery to thin the endometrium and prevent excessive blood loss during the procedure. Danazol is given in doses of 100-200 mg per day for 3 months.
Non-hormonal Therapies. Nonsteroidal anti-inflammatory drugs (NSAIDs) act by inhibiting prostaglandin synthesis. They reduce the elevated endometrial prostaglandin levels seen in women with menorrhagia. NSAIDs are generally useful for the treatment of ovulatory menorrhagia. Common NSAIDs used are mefenamic acid, naproxen sodium, and ibuprofen. They are given daily until bleeding improves, and then given at the start of menstruation as needed.
Tranexamic acid is an oral antifibrinolytic agent that acts by inhibiting the degradation of fibrin. It is a relatively new medication that is very effective for treatment of DUB.11 The exact dosage varies with each individual, and is given 3-4 times per day for the first 4 days of the menstrual cycle.
Emergency physicians are more likely to intervene in patients with acute, heavy bleeding, and high-dose estrogen therapy is the treatment of choice for these patients. Options for high-dose estrogen administration are listed below, with no significant difference between onset of action for intravenous or oral administration:
Twenty-five milligrams conjugated estrogen intravenously every 3-4 hours. Several doses are usually needed, and surgical intervention should be considered if bleeding is not controlled within 24 hours.
Ten milligrams of oral conjugated estrogen in four daily doses, for 24-48 hours. The dose should be doubled to 20 mg a day if unsuccessful. High-dose oral contraceptive using a monophasic combination pill containing 35 μg ethinyl estradiol is given 3 to 4 times per day in divided doses. The pills are continued for a week following cessation of bleeding.
If bleeding is stopped with estrogen therapy alone, a progestin, such as medroxyprogesterone acetate 10 mg per day, should be added for 7-10 days. The medication is then stopped to allow withdrawal bleeding to occur. Regular oral contraception is recommended for 3-4 cycles after this type of intervention. Patients requiring high-dose estrogen therapy for acute vaginal bleeding should usually be admitted to the hospital for observation.
Surgical Intervention. In cases unresponsive to medical therapy, surgical intervention may be performed. Dilation and curettage is the quickest method to stop significant acute bleeding. Endometrial laser ablation and hysteroscopic transcervical resection of the endometrium (HRTE) are additional methods used to surgically treat DUB. HRTE uses electrocautery to coagulate and remove the endometrium and may prevent the need for hysterectomy. The most definitive surgical treatment is hysterectomy.
The decision to start treatment in the ED depends on the severity of bleeding and anticipated compliance with medication and follow-up. The decision should be undertaken in conjunction with the consulting gynecologist.
Postmenopausal Vaginal Bleeding
Postmenopausal women who visit the ED for vaginal bleeding may have a variety of benign conditions unrelated to menopause, bleeding as a result of the hormonal changes associated with menopause, or an underlying malignancy. Approximately 10% of postmenopausal women will experience vaginal bleeding.12 The biggest concern is uterine cancer, which cannot be diagnosed by history and physical exam alone. It is estimated that only about 5% of women with this presentation will have endometrial cancer.12 The most common cause of postmenopausal bleeding is vaginal or endometrial atrophy. A lack of estrogen causes dry endometrial or mucosal surfaces that tend to become inflamed and bleed. Bleeding is usually light. Other common causes include bleeding secondary to endometrial hyperplasia, postmenopausal hormone replacement therapy (HRT), structural lesions, and anticoagulant use. Endometrial hyperplasia is abnormal in the postmenopausal woman with estrogen deficiency and must be investigated. Exogenous estrogen therapy, obesity, or estrogen-producing tumors of the ovary or adrenal gland may be causative. Structural lesions are less common in menopause, but may occur. It is important to note that uterine cancer may coexist with a structural lesion such as a leiomyoma or polyp, and the investigation into the cause of bleeding must always be to exclude the possibility of cancer.
Bleeding from atrophic conditions of the vagina or endometrium, HRT, and early uterine cancer is usually light. On physical exam, non-vaginal causes of bleeding from urological and gastrointestinal sources are excluded. The gynecologic exam is usually normal, but atrophic vaginal mucosa and ulceration may be seen. It is common to check a complete blood count and, if the patient is on warfarin, an INR. Blood for type and crossmatch should be sent if transfusion is anticipated. The definitive ancillary testing for bleeding in this age group is not made in the ED, and the decision to admit or discharge depends on whether the bleeding is under control.
Genital Tract Pathology
Genital tract pathology that may present with vaginal bleeding can be divided into several broad categories: structural, infectious, and malignant. History, physical exam, and ancillary testing, combined with knowledge of predisposing factors, are key to establishing the correct diagnosis.
Structural Pathology
Uterine leiomyomas (fibroids) are the most common structural lesions of the genital tract, occurring in up to 30% of women of reproductive age.13 They are benign, estrogen-dependent tumors originating from the smooth muscle of the uterus, and are most commonly discovered in women between the ages of 30 and 45. The etiology of these tumors is unknown, but there is a predilection for African-American women, who have a three times greater incidence of fibroids than Caucasian women.14 In addition, increased risk for fibroids is seen in patients with a history of hypertension, diabetes, nulliparity, obesity, POC, and those with a family history.15 Women who are symptomatic from their fibroids most commonly present with menorrhagia; fatigue, iron deficiency anemia, and pain are often present. Intermenstrual bleeding is not a common symptom of fibroids. Location is more of a predictor of bleeding than size, and submucosal fibroids are implicated as the most common cause of irregular bleeding.16 Fibroids can be large and significantly distort the uterus, and are palpable on physical exam. For convention, this type of uterine enlargement is often described in gestational weeks, similar to a gravid uterus. For example, a fibroid uterus that reaches the umbilicus would be described as 20 weeks size. Patients with symptomatic fibroids usually undergo elective hysterectomy, myomectomy, or uterine artery embolization. GnRH analogues are used to reduce the size of fibroids pre-operatively.17 GnRH agonists, progesterone-receptor modulators such as mifepristone and asoprisnil, selective estrogen-receptor modulators, and androgens may be useful non-surgical therapies to reduce menstrual blood loss and fibroid size.18,19,20
Figure 2: Leiomyoma of the Cervix

Transvaginal ultrasound reveals a large, solid, inhomogeneous mass surrounded by fluid in the endocervical canal on transverse (left) and sagittal (right) imaging. These findings are consistent with a leiomyoma.
Image reproduced with permission from Dr. Joe Antony ©http://www.ultrasound-images.com.
In the ED, a presumptive diagnosis of uterine fibroids can be made in the patient who presents with vaginal bleeding and an enlarged, irregular, and mobile uterus on abdominal or bimanual exam. In the patient with no history of fibroids, a transabdominal or transvaginal ultrasound can be done to confirm the diagnosis and to exclude other causes. (See Figure 2.) Further work-up includes orthostatic vital signs, a complete pelvic exam, a complete blood count to evaluate for significant anemia, and coagulation studies if a coagulopathy is suspected or the patient is on warfarin. If the work-up is normal and the patient's bleeding is mild to moderate, she may be discharged with close follow-up with a gynecologist. Oral iron therapy may be indicated to counter the effects of ongoing blood loss. In the hemodynamically unstable patient, or the patient with potentially life-threatening blood loss, initial ED stabilization follows standard protocol for the unstable patient, including large-bore intravenous access, fluid bolus, blood transfusion, and emergent gynecologic consultation. If uncontrolled hemorrhage continues, the patient should be prepped for emergency hysterectomy.
Adenomyosis is a disorder in which ectopic endometrial stoma and glandular tissue become embedded deep within the myometrium of the uterus. This tissue causes hypertrophy of the surrounding myometrium and enlargement of the uterus. The ectopic tissue is either spread throughout the myometrium or forms focal masses like those seen with fibroids. The pathophysiology of this disorder is unknown. The classically described presentation occurs in older reproductive-age women who experience cyclic, crampy pain and heavy menstrual bleeding. Newer literature shows this disease may be more prevalent than previously thought and is found in younger women as well, often coexistant with fibroids and endometriosis. Intermenstrual bleeding may also occur. The physical exam may reveal bleeding and an enlarged, tender uterus. Uterine enlargement does not usually exceed 12 to 14 weeks size, which may distinguish it from fibroids in some cases.
In the ED, making a definitive diagnosis of adenomyosis is both unnecessary and difficult. The disease may be suspected in a patient with menorrhagia who has an enlarged, soft, but tender uterus. Ultrasound may show a diffuse infiltrative process, but may not distinguish adenomyosis from a more serious condition such as endometrial carcinoma. MRI is the diagnostic modality of choice. Elective treatment for this disease is focused on symptomatic relief of pain and bleeding. Hormonal treatment has varied success, and hysterectomy is curative.
Emergency management of the unstable patient is the same as that for fibroids.
Polyps refer to the growth of tissue projecting from a mucosal surface. In the reproductive tract, they occur most commonly on the cervix or endometrium and may cause vaginal bleeding. Although the etiology of polyps is unclear, hypertension and obesity are considered the major risk factors. Polyps are most commonly found in women of reproductive age. The incidence increases with age, with most cases occurring in women in their 40s, and few cases diagnosed before the age of 20. Endometrial polyps are varied, ranging from single to multiple and small to large enough to protrude through the cervical canal. Unlike cervical polyps, these are not visible on physical exam unless they have prolapsed through the cervical canal and, therefore, are more difficult to diagnose. Malignant transformation is rare. Cervical polyps usually present with postcoital and inter-menstrual bleeding and do not cause significant hemorrhage. They most commonly arise from the endocervix as soft, fleshy, pedunculated masses protruding from the cervical canal. They may also arise from the ectocervix as broader-based masses that do not tend to bleed. Endocervical polyps are usually small, between 1 and 2 cm in length, and may not be palpable on bimanual exam. Unless they are high in the endocervical canal, however, most polyps are visible on speculum exam. They often are inflamed and may appear as necrotic masses arising from the cervix. Although cervical polyps have a low incidence of malignant transformation, if they are atypical in appearance, they must be differentiated from endocervical malignancies. Postmenopausal women with polyps have a higher incidence of malignant transformation and should undergo biopsy.21 It is customary to remove cervical polyps for treatment and histological examination, a practice which has been recently questioned.22
Imaging is usually ordered from the ED when bleeding is significant and the etiology is unknown. Transvaginal ultrasound has a sensitivity and specificity of approximately 43% and 74%, respectively, for diagnosing endometrial polyps when compared to pathology reports. The comparable values of 76% and 81% with a technique called hysterosonography make it the diagnostic test of choice.23 Hysterosonoraphy is performed by instilling fluid into the endometrial canal before ultrasound evaluation, which delineates the endometrial cavity with more accuracy. No imaging distinguishes benign from malignant disease, and further evaluation by a gynecologist is indicated. Treatment of significant bleeding follows the same principles as with other structural lesions. In most circumstances, the role of the ED physician is to assure appropriate follow-up.
Endometriosis is a disease characterized by endometrial glandular tissue and stroma located outside the uterus and myometrium. Although the exact etiology is unknown, the most popular theory involves retrograde flow of menstrual blood through the fallopian tubes, with subsequent dissemination of endometrial tissue outside the uterus. Risk factors include a family history and anatomic defects that cause outflow obstruction of the reproductive tract. Several studies have linked an exposure to environmental toxins and the development of endometriosis, but a recent publication disputes this claim.24 Endometriosis most commonly occurs on the ovaries ("chocolate cysts"), but may be found in other areas of the pelvis, such as the fallopian tubes and urinary tracts. Extrapelvic disease may occur in almost any other organ system, and cases involving the pulmonary, gastrointestinal, and central nervous system have been published.25,26,27 Because the definitive diagnosis is made at laparoscopy, and many women are asymptomatic, the exact incidence is unknown. It has a general prevalence of approximately 5%. In women with infertility, the rate increases to 20-40%.28 Endometriosis is dependent on estrogen and is, therefore, most commonly seen in women during their reproductive years.29 It often causes debilitating pelvic pain, dysmenorrhea, and infertility. Intermenstrual bleeding and menorrhagia are the most common forms of bleeding, but rarely, significant hemorrhage can occur.30 Patients with endometriosis present with a variety of complaints, depending on the location of the disease. Non-localizing pelvic tenderness is common. Adnexal masses or nodular masses on the posterior uterus and posterior cul-de-sac may be palpable. When patients present with bleeding, the physical exam is usually non-specific, and the etiology of vaginal bleeding cannot be determined by exam alone. In addition, there are no specific laboratory tests to aid in this diagnosis. Emergent ultrasonography is not indicated unless another more serious cause of vaginal bleeding is suspected. Stable patients can be discharged with close follow-up for outpatient evaluation. Pain medication may be indicated.
The mainstay of medical treatment is hormonal therapy, which should be prescribed by the supervising gynecologist on an outpatient basis.
Infectious Pathology
In most cases, the history and physical exam will be diagnostic of an infectious process. Endometritis often presents acutely as a complication of vaginal delivery, but may occur after intrauterine device (IUD) insertion or with pelvic inflammatory disease. Predisposing conditions include history of endometrial biopsy or dilatation and curettage, and uterine leiomyomas. Endometritis may also occur in postmenopausal women who have an underlying malignancy. Vaginal bleeding, purulent discharge, pelvic pain, fever, and uterine tenderness are common. A chronic form of endometritis may occur without fever, and with varying types of vaginal bleeding.31 Menorrhagia or metrorrhagia, intermenstrual bleeding, and postcoital bleeding may occur. Vaginitis and cervicitis may also cause vaginal bleeding that is apparent on speculum exam. Bleeding is from local mucosal irritation and is usually light. Treatment of the underlying infection should resolve the bleeding.
Malignant Pathology
Although any malignancy involving the reproductive tract can cause bleeding, uterine and cervical cancer are the most common, and will be discussed. Less common malignancies that cause bleeding are vulvar, vaginal (very rare), ovarian, and fallopian tube cancers.
Uterine cancer is the most common gynecological malignancy. In 2007, 41,314 women in the United States were diagnosed with uterine cancer, and 7,456 died from the disease.32 The etiology is unknown, but exposure to exogenous and endogenous estrogens is considered to have a causal relationship to carcinogenesis.33 Risk factors include a family history of the disease, medications such as unopposed estrogens and tamoxifen, obesity, age greater than 50 years, and conditions supporting unopposed endogenous estrogen, such as nulliparity, advanced age at first birth, and menopause. A condition known as hereditary nonpolyposis colon cancer is associated with a significantly increased risk of uterine cancer. Uterine cancer is twice as common in Caucasian women as African-American women.34 Risk of uterine cancer increases with age, and the majority of cases are diagnosed in the postmenopausal woman. Twenty-five percent of the cases occur in premenopausal women, with 5% in women younger than 40 years of age.35 Unfortunately, there is no screening for this disease in asymptomatic women. The most common presentation in uterine cancer is abnormal vaginal bleeding, and should be considered in every woman who presents with this complaint. Bleeding may be menorrhagia, metrorrhagia, or any pattern of bleeding in the postmenopausal patient. It must be remembered that the amount of bleeding is not an indicator of the severity of the disease, and even spotting is concerning. While a PAP smear is not a screening test for uterine cancer, the presence of endometrial cells warrants further investigation in the postmenopausal patient.
The physical exam in uterine cancer is usually normal, and bleeding, if present, is usually not life-threatening. No specific laboratory abnormality aids in this diagnosis. An ultrasound can be done in the ED to exclude other forms of gynecological pathology. A thickened endometrium on transvaginal ultrasound, as seen in Figure 3, is suspicious, and is never normal in the postmenopausal patient. The stable patient can be referred for outpatient work-up if there is no significant anemia. Patients on warfarin should have their INR checked and reversal of anticoagulation or alteration in dosage if indicated. In the unstable patient, emergent resuscitation and gynecologic consultation should be instituted. The main treatment for uterine cancer is surgical.
Figure 3: Ultrasound Image of Thickened Endometrium

Transvaginal ultrasound in a postmenopausal patient shows a grossly thickened endometrium measuring 2.83 by 3.18 cm. Histopathological exam revealed adenocarcinoma.
Image reproduced with permission from Dr. Joe Antony ©http://www.ultrasound-images.com.
Cervical cancer is a preventable disease with an incidence that has been declining in the United States during the past 40 years due to screening PAP smears. In 2007, 12,280 women in the United States were diagnosed with the disease, with 4,021 deaths during that year.32 In developing countries, however, where screening is not routinely performed, the disease is much more prevalent. The World Health Organization's statistics identify 85% of the cervical cancer burden on women in developing countries, where cervical cancer accounts for 13% of all female cancers.36 Cervical cancer is associated with sexual activity. Infection with human papillomavirus (HPV) is the most important risk factor.37 Cervical cancer occurs in about 20% of patients who have persistent HPV infection, most commonly with HPV serotypes 16 and 18.38 HPV testing may be more sensitive than PAP smears for cervical cancer.39 Other reported risk factors include smoking, immunocompromised states, long-term oral contraceptive use, and multiple births.40 The development of a vaccine against the major serotypes of HPV related to cervical cancer is a promising start to prevent this disease.
Most cervical cancers are discovered on routine PAP smear before the patient is symptomatic. Patients often present to the ED when the disease is more advanced. The most common symptom is vaginal bleeding. While postcoital bleeding has been described as the hallmark of the disease, only a small subset of patients with that complaint will have cervical cancer.41 Metrorrhagia, intermenstrual bleeding, and postmenopausal bleeding are more common. Because cervical cancer spreads initially by local extension to adjacent organs and lymph nodes, patients with advanced disease may present to the ED with pelvic pain, urinary symptoms, and lower extremity edema. The physical exam should include a visual inspection of the cervix and a thorough bimanual, including a rectovaginal exam, to palpate for local masses or adhesions. The appearance of the cervix may be varied and can be confused for benign conditions such as cervicitis or cervical erosion (ectropion). Lesions may be ulcerative, friable, and necrotic, or may present as a visible mass extending from the exocervix. Local extension into the vaginal vault may occur.
In the stable patient, the diagnosis of cervical cancer is made on an outpatient basis. Treatment options include surgery, radiation, and chemotherapy or combination therapy.
Reproductive Tract Trauma
Injury to the labia or vagina is the most common genital tract injury in women. It occurs secondary to trauma or vigorous sexual activity, and must be considered in the patient who presents with vaginal bleeding in this setting. Pain often accompanies the bleeding. Sexual abuse can present with similar clinical findings. A high index of suspicion is essential to identify the victim of sexual assault or abuse. Studies attempting to correlate the type of genital injury specific for sexual abuse have not been conclusive.42
Trauma to the genital tract may be accidental or intentional. Mechanisms of injury include blunt trauma caused by motor vehicle accidents, or physical assault, sexual assault, vigorous consensual intercourse, falls, and penetrating injury. Obstetrical causes of genital trauma are common. Patients with lower abdominal or pelvic trauma are at high risk for genital injury and should always undergo a thorough vaginal examination. Indirect trauma to the genital tract may occur from injuries such as pelvic fractures, in which bony fragments perforate the vagina and present with vaginal bleeding. A less common but reported injury occurs in patients participating in water sports, such as jet- or water-skiing and water chutes, in which high pressure insufflation vaginal injuries occur. In this circumstance, pressurized water over-distends the vagina and may result in vaginal tears.
The most common injuries to the labia or vagina are lacerations and hematomas, which are usually evident on genital exam. A careful speculum examination must be included, as some types of internal genital injuries may present with normal-appearing external genitalia. Antibiotics are often given in significant wounds, although their use is controversial. Tetanus prophylaxis should be given when indicated.
Labial injuries are usually the result of blunt trauma and are often seen in straddle-type injuries, especially in younger adults. The perineum has a rich vascular supply, making it susceptible to bleeding. The fat pads present in the labia of adult females, however, protect them from injury, which is why hematomas are more common than lacerations in this area. The diagnosis of a vulvar hematoma is usually evident from inspection of the labia. Fluctuance, swelling, and pain of the involved labia are indicative of hematoma formation. A thorough vaginal and rectal exam must be performed to assess for concurrent injury to adjacent structures. If the patient cannot cooperate with the exam, it should be accomplished with sedation or general anesthesia. Complications associated with vulvar hematomas include voiding problems if the hematoma compresses the urethra, associated vaginal or rectal trauma, infection, and hematoma expansion that may result in hemodynamic instability. Bladder catheterization may be required. As most hematomas are of venous origin, management of uncomplicated hematomas is conservative, with compressive dressings, NSAIDs, and rest. In the majority of patients with non-expanding hematomas, non-operative intervention is successful.43 Hemodynamic instability or a dropping hematocrit should raise concern for an expanding extraperitoneal hematoma and may require surgical intervention. Incision and drainage of the hematoma, ligations of identified bleeding arteries, simple closure, or placement of a drain to prevent recurrent hematoma formation is the customary treatment. Transarterial embolization has been utilized, but is extremely rare.44
Vaginal injuries are more commonly due to penetrating trauma and usually result in vaginal lacerations. This may occur from vigorous consensual intercourse or sexual abuse, foreign body penetration, pelvic trauma, or water sports-related injury. A speculum exam must be thorough and provide visualization of all portions of the vaginal vault, including the distal vaginal wall as the speculum is removed. If necessary, sedation or examination under anesthesia should be utilized.
The most common site for vaginal lacerations from sexual abuse or intercourse is the posterior fornix and lateral walls of the vagina. A digital rectal exam should be performed to determine if any mucosal disruption exists between the rectum and posterior vaginal wall. Minor lacerations with no active bleeding limited to superficial mucosa or submucosal tissue can be left to heal on their own. If the laceration is minor and superficial but fails to achieve adequate hemostasis, packing with sterile saline-soaked or estrogen cream-impregnated gauze may be all that is needed. Lacerations that continue to bleed require surgical repair, which should be done by a gynecologist or surgeon to avoid injury to the urethra, bladder, or rectum. On rare occasions, a vaginal laceration can extend into the peritoneal cavity.45 If vaginal bleeding is excessive and prevents visualization of the vaginal wall, or if the patient is unstable, the vaginal vault should be packed with sterile gauze to temporarily tamponade the bleeding prior to definitive management. The patient should be taken to the operating room where adequate visualization and laceration repair can occur. Complications from vaginal lacerations include infection, associated lower urinary tract injury, or rectal injury. If inappropriately treated, fistula formation between the vagina and bladder or bowel may occur.
Non-gynecological Causes
Clinical conditions that may cause excessive vaginal bleeding include chronic renal failure, uremia, idiopathic thrombocytopenic purpura, leukemia, factor deficiencies, and chronic liver disease. Bleeding may be caused by medications, such as anticoagulants and hormones. OCPs may be associated with amenorrhea, menorrhagia, or intermenstrual bleeding. Endocrine disorders such as hyper- or hypothyroidism, hyperprolactinemia, Cushing disease, Addison disease, and ovarian tumors may also be associated with bleeding. In adolescents, heavy menstrual bleeding may be the first clue to an undiscovered congenital bleeding disorder such as von Willebrand's disease; the astute clinician considers bleeding disorders in this group of patients. If anatomic pathology is excluded, screening clotting assays should be performed.
A detailed past medical, family, and medication history may provide the clue to the cause of bleeding from non-gynecologic sources. Treatment is aimed at correcting the underlying disorder.
Imaging Modalities in Vaginal Bleeding
Ultrasound. Ultrasound has the advantage of being noninvasive and readily available in most institutions. It has limitations in the obese patient. Ultrasound is the most common imaging used in the ED to investigate the cause of vaginal bleeding and is an excellent modality to visualize the endometrial lining and myometrium. Normal cut-off values for endometrial thickness on transvaginal sonography have been reported to be less than 5 mm in postmenopausal women and less than 16 mm in premenopausal women.46,47 The finding of a thickened endometrial lining by transvaginal ultrasound is sensitive but not specific for endometrial disease. Also, in patients with genital trauma and labial swelling, ultrasound may distinguish hematoma from abscess formation. Masses seen on ultrasound may be indeterminate and require further imaging to distinguish benign from malignant lesions.
A CT scan is best used in the ED when non-gynecologic pathology is entertained in the differential diagnosis. MRI is most useful in the diagnosis of adnexal or extra-adnexal masses that are indeterminate on ultrasound. MRI is preferred over CT for this purpose.48 Most information needed for ED management and disposition of the patient can be made using ultrasound.
References
1. Liu Z, Doan QV, Blumenthal P, Dubois RW. A systematic review evaluating health-related quality of life, work impairment, and health-care costs and utilization in abnormal uterine bleeding. Value Health 2007;10:183-194.
2. Bushnell D, Martin M, Moore K, et al. Menorrhagia Impact Questionnaire: Assessing the influence of heavy menstrual bleeding on quality of life. Current Medical Research and Opinion 2010;26:2745-2755.
3. Woolcock J, Critchley H, Munro M, et al. Review of the confusion in current and historical terminology and definitions for disturbances of menstrual bleeding. Fertil Steril 2008;90:2269-2280.
4. Chimbira TH, Anderson ABM, Turnbull AC. Relation between measured menstrual blood loss and patient's subjective assessment of loss, duration of bleeding, number of sanitary towels used, uterine weight and endometrial surface area. International J Obstet Gynaecol 2005;87:603–609. doi: 10.1111/j.1471-0528.1980.tb05013.x
5. Maness D, Reddy M, Harraway-Smith C, et al. How best to manage dysfunctional uterine bleeding. J Family Practice 2010;59:449–458.
6. Sanfilippo J, Lara-Torre E. Adolescent Gynecology. Obstetrics & Gynecology 2009;113:935–947.
7. Santoro N, Crawford S, Lasley W, et al. Factors related to declining luteal function in women during the menopausal transition. J Clin Endocrinology & Metabolism 2008;93:1711-1721.
8. Van Voorhis B, Santoro N, Harlow Sioban, et al. The relationship of bleeding patterns to daily reproductive hormones in women approaching menopause. Obstetrics & Gynecology 2008;112:101–108.
9. Frazier JK, Smith C. "Vaginal Bleeding." In: Mengel M, Schweibert P, eds. Family Medicine: Ambulatory Care and Prevention. New York: McGraw Hill; 2009:445–448.
10. Hale G, Hughes C, Burger H, et al. Atypical estradiol secretion and ovulation patterns caused by luteal out-of-phase (LOOP) events underlying irregular ovulatory menstrual cycles in the menopausal transition. Menopause 2009;16:50-59.
11. Lukes A, Andrea S, Moore K, et al. Tranexamic acid treatment for heavy menstrual bleeding: A randomized controlled trial. Obstetrics & Gynecology 2010;116:865-875.
12. Marshburn B, Hurst B, Eds. Disorders of Menstruation. Hoboken, NJ: Wiley-Blackwell; 2011.
13. Fife R, Schrager S, eds. ACP Handbook of Women's Health. American College of Physicians: Philadelphia, PA; 2009.
14. Van Voorhis B. A 41-year-old woman with menorrhagia, anemia, and fibroids. JAMA 2009;301:82-93.
15. Okolo S. Incidence, etiology and epidemiology of uterine fibroids. Clinical Obstetrics & Gynaecology 2008;22:571–588.
16. Salman T, Davis C. Uterine fibroids, management and effect on fertility. Curr Opin Obstetrics & Gynecology 2010;22:295–303.
17. Management of Uterine Fibroids: An Update of the Evidence. Structured Abstract. July 2007. Agency for Healthcare Research and Quality, Rockville, MD. http://www.ahrq.gov/clinic/tp/uteruptp.htm.
18. Engman M, Granberg S, Williams AR, et al. Mifepristone for treatment of uterine leiomyoma. A prospective randomized placebo controlled trial. Hum Reprod 2009;24:1870-1879.
19. Wilkens J, Chwalisz K, Han C, et al. Effects of the selective progesterone receptor modulator asoprisnil on uterine artery blood flow, ovarian activity, and clinical symptoms in patients with uterine leiomyomata scheduled for hysterectomy. J Clin Endocrinol Metab 2008;93:4664-4671.
20. Parsanezhad ME, Azmoon M, Alborzi S, et al. A randomized, controlled clinical trial comparing the effects of aromatase inhibitor (Letrozole) and gonadotropin-releasing hormone agonist (Triptorelin) on uterine leiomyoma volume and hormonal status. Fertil Steril 2010;93:192-198.
21. Antunes A, Costa-Paiva L, Arthuso M, et al. Endometrial polyps in pre- and postmenopausal women: Factors associated with malignancy. Maturitas 2007;20;57:415-421.
22. MacKenzie Z, Naish C, Rees CMP, et al. Why remove all cervical polyps and examine them hystiologically? BJOG 2009;116:1127–1129.
23. Paszkowska, M, Torres, A, Paszkowski, M, et al. Can we rely on transvaginal ultrasonography in the detection of endometrial polyps? A comparison of transvaginal ultrasonography, hysteroscopy and pathology reports. Ultrasound in Obstetrics & Gynecology 34:284–285.
24. Guo SY, Simsa P, Kyama C, et al. Reassessing the evidence for the link between dioxin and endometriosis: From molecular biology to clinical epidemiology. Mol Hum Reprod 2009;15:609-624.
25. Channabasavaiah A, Joseph J. Thoracic endometriosis: Revisiting the association between clinical presentation and thoracic pathology based on thoracoscopic findings in 110 patients. Medicine 2010;89:183–188.
26. De Ceglie A, Bilardi C, Blanchi S, et al. Acute small bowel obstruction caused by endometriosis: A case report and review of the literature. World J Gastroenterol 2008;14:3430–3434.
27. Sarma D, Iyengar P, Marotta TR, et al. Cerebellar endometriosis. AJR Am J Roentgenol 2004;182:1543-1546.
28. Ozkan, S, Murk W, Arici A. Endometriosis and infertility. Ann New York Academy of Sciences 2008;1127:92–100.
29. Bulun S. Endometriosis. N Engl J Med 2009;360:268-279.
30. Wong F., Lim C, Karia S, et al. Cervical endometriosis: Case series and review of literature. J Obstet Gynaecol Res 2010; 36: 916–919.
31. Cicinelli E, DeZiegler D, Nicoletti R, et al. Poor reliability of vaginal and endocervical cultures for evaluating microbiology of endometrial cavity in women with chronic endometritis. Gynecol Obstet Invest 2009;68:108-115.
32. U.S. Cancer Statistics Working Group. United States Cancer Statistics: 1999–2007 Incidence and Mortality Web-based Report. Atlanta (GA): Department of Health and Human Services, Centers for Disease Control and Prevention, and National Cancer Institute; 2010. http://www.cdc.gov/uscs.
33. Jensen A, Sharif H, Kjaer S. Use of fertility drugs and risk of uterine cancer: Results from a large Danish population-based cohort study. Am J Epidemiol 2009;170:1408-1414.
34. Sorosky J. Endometrial cancer. Obstetrics & Gynecology 2008;111:436-447.
35. Muggia F, Oliva E, ed. Uterine cancer: Screening, Diagnosis, and Treatment. New York, NY: Springer; 2009.
36. Ferlay J, Shin HR, Bray F, et al. GLOBOCAN 2008, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 10 [Internet]. Lyon, France: International Agency for Research on Cancer; 2010. Available from: http://globocan.iarc.fr.
37. Schiffman M, Castle P, Jeronimo J, et al. Human papillomavirus and cervical cancer. Lancet 2007;370:890-907.
38. International Collaboration of Epidemiological Studies of Cervical Cancer. Cervical carcinoma and sexual behavior: Collaborative reanalysis of individual data on 15,461 women with cervical carcinoma and 29,164 women without cervical carcinoma from 21 epidemiological studies. Int J Cancer 2007;120: 885-891.
39. Mayrand M, Duarte-Franco E, Rodrigues I, et al. Human papillomavirus DNA versus Papanicolaou screening tests for cervical cancer. N Engl J Med 2007;357:1579-1588.
40. Louie K, Sanjose S, Castellsague X, et al. Early age at first sexual intercourse and early pregnancy are risk factors for cervical cancer in developing countries. Br J Cancer 2009;100:1191–1197.
41. Shapely M, Jordan J. A systematic review of postcoital bleeding and risk of cervical cancer. Br J Gen Pract 2006;56(527):453–460.
42. Merritt D. Vulvar and genital trauma in pediatric and adolescent gynecology. Curr Opin Obstetrics & Gynecology 2004;16:371-381.
43. Propst A, Thorp J. Traumatic vulvar hematomas: Conservative versus surgical management. South Med J 1998;9:144-146.
44. Kunishima K, Takao H, Kato N, et al. Transarterial embolization of a nonpuerperal traumatic vulvar hematoma. Radiation Medicine 2008;26:168-170.
45. Jones J, Worthington T. Genital and anal injuries requiring surgical repair in females less than 21 years of age. J Pediatr Adolesc Gynecology 2008;21:207-211.
46. Skaznik-Wikiel M, Malgorzata E, Jelovsek, J, et al. Accuracy of endometrial thickness in detecting benign endometrial pathology in postmenopausal women. Menopause 2010; 17: 104-108.
47. Iyer V, Lee S. Gynecologic imagining: Current and emerging applications. Symposium 2010;56:117-124.
48. Kinkel K, Lu Y, Mehdizade A, et al. Indeterminate ovarian mass at US: Incremental value of second imaging test for characterization-meta-analysis and Bayesian analysis. Radiology 2005;236:85-94.
Abnormal uterine bleeding affects females of all ages and is a common complaint in gynecologic practice. An estimated $1 billion annually is spent on direct costs for this diagnosis, with another $12 billion attributed to indirect costs such as health-related supplies and missed employment.Subscribe Now for Access
You have reached your article limit for the month. We hope you found our articles both enjoyable and insightful. For information on new subscriptions, product trials, alternative billing arrangements or group and site discounts please call 800-688-2421. We look forward to having you as a long-term member of the Relias Media community.
