Fever in Infants < 3 Months Old: What is the Current Standard?
Authors:
Dennis A. Hernandez, MD, FAAP, FACEP, FAAEM, Medical Director, Pediatric Emergency Services, Florida Hospital for Children, Walt Disney Pavilion, Orlando, FL
Vu Nguyen, MD, Resident, Florida Hospital Emergency Medicine Residency, Orlando, FL
Peer Reviewer:
Brian S. Skrainka, MD, FAAP, FACEP, Children's Medical Center (of Dallas) at Legacy, Plano, TX
Fever has been recognized as a symptom of illness for centuries. It is especially concerning when it is associated with a young infant. The febrile infant has been the subject of much debate and research, especially in the last few decades. This drive to discover the best management strategy led to the publication of practice guidelines in 1993. Many advances have occurred in the last two decades, notably, the availability of the Haemophilus influenzae type B (Hib) and pneumococcal vaccines.1
Although most febrile neonates and young infants have self-limiting viral infections, the aim of practice guidelines is to identify those infants who are at increased risk for serious bacterial infection (SBI) (i.e., bacterial meningitis, pneumonia, bacteremia, urinary tract infection, bacterial gastroenteritis) and therefore who will benefit from hospitalization and perhaps empiric treatment.2
Unfortunately, due to maturational immunologic deficiencies and a lack of effective communication, neonates and young infants may demonstrate few, if any, clinical clues to an underlying illness.3,4 Many times, fever may be the only presenting symptom. The incidence of SBI is relatively high in febrile infants, particularly in those < 28 days, as compared to older children.5 To clinically discern which infant is harboring an SBI is difficult to impossible when the infant is well-appearing. This has led clinicians to develop an aggressive approach in managing febrile infants, including diagnostic tests and, frequently, hospital admission pending culture results. However, a "shotgun" approach in every child can be costly and has the added risk of complications from medications and diagnostic testing.
Because of the increasing problem with drug resistance, iatrogenic problems, and the relatively low incidence of SBIs in febrile neonates, efforts have been made to see if any specific tests can allow febrile neonates with low-risk factors to be observed without use of antibiotics and hospitalization.6 Although some studies have shown that febrile neonates who fit low-risk criteria can be managed without antibiotics, hospitalization and close observation still are required at a minimum.7
Although little disagreement exists regarding the current management of neonates younger than 30 days, considerable disagreement and practice variation exists for children 31-90 days old.8
In light of this, the emergency department (ED) physician is faced with a number of significant decisions in the management of these patients, having to consider patients' inability to communicate, their immune system development, immunization status, and parental perception of seriousness.8 This article explores the controversies associated with the febrile infant to discern current recommendations.
Ann Dietrich, MD, FAAP, FACEP
Introduction
Definition of Fever. Fever in infants traditionally has been defined as a temperature of ≥ 38° C (100.4° F) measured rectally. This cutoff may be slightly higher depending on the study cited and local institution. Although axillary and tympanic measurements are convenient, they are not reliable in young infants and rectal temperatures remain the standard for detecting fever.4 In any case, temperature should not be the only factor used to make a management decision. This is particularly important if the infant is afebrile but has a history of irritability, poor feeding, lethargy, or any other behavioral change that is of concern to the parent.4
Serious Bacterial Infections. The utility of many studies and protocols is measured by their ability to identify or prevent SBIs. Most studies have defined SBIs as bacterial meningitis, bacteremia, bacterial pneumonia, soft tissue and skin infections (i.e., cellulitis, omphalitis), osteomyelitis, bacterial gastroenteritis, septic arthritis, or urinary tract infection (UTI).5
The incidence of SBI reported can vary depending on the study cited due to the variability in defining parameters. A meta-analysis of studies published between 1974 and 1990 reported an SBI incidence of 7% in febrile infants (> 39° C) under 3 months of age with bacteremia and bacterial meningitis occurring in 2.5%; a higher incidence of infections occurred in infants < 1 month of age (8.7% and 3.7%, respectively).9
In infants < 3 months of age, UTI accounts for about one-third of all bacterial diseases with current data suggesting the rate of bacteremia to be between 2%-3% in all febrile infants under 2 months of age.10 Despite the decrease in incidence, SBI continues to be a concern in young infants, especially in the first month of life.
Bacterial Pathogens. Historically, before the advent of the Hib vaccine, the most common pathogens of bacteremia included S. Pneumoniae (65%-75%), H. influenzae Type b (Hib, 10%-20%), N. meningitidis (5-15%), and Salmonella sp. (5%-15%). Pathogens like Streptococci group B, E. coli, and L. monocytogenes are especially more common among neonates. Although S. pneumoniae was the most common culprit of occult bacteremia, Hib tends to follow a more invasive and dangerous course of disease.10 However, since the introduction and widespread use of the Hib vaccine in the early 1990s, the prevalence of Hib disease and its sequelae virtually has been eliminated. Recent studies on occult bacteremia have reported the incidence of occult bacteremia in young children with fever to be 1.6%-1.9%; with pneumococcus representing 83%-92% of these cases.11,12
The licensing of the heptavalent pneumococcal conjugate vaccine (PCV7) in 2000, and more recently PCV13, also has changed the dynamic of invasive bacterial disease. Since this licensing, in a study involving eight U.S. children's hospitals, the rate of invasive pneumococcal infection has decreased more than 75% in children younger than 24 months.13 A post-licensure re-examination of the Northern California Kaiser Permanente study cohort demonstrated a decline in invasive pneumococcal disease of 94% in children less than 1 year of age.14,15
Protocols
Studies have been conducted in an effort to identify patients who are low risk for bacterial infections and can be managed safely as outpatients with or without empiric antibiotic treatment.16-26 These studies varied in their patient population, inclusion/exclusion criteria, and uniformity in workup and management. This had led to a lack of consensus and debate concerning the best approach to care for the febrile infant. Despite the established guidelines, variability in application exists among many practitioners.27-29
Nonetheless, these studies established that certain infants can be managed safely as outpatients and can avoid unnecessary hospitalization.26 The incidence of serious bacterial infection among infants categorized as low-risk after appropriate evaluation ranged from 0.5%-1.1% in studies that utilized or excluded the lumbar puncture as part of the initial evaluation, respectively.30 The Boston, Rochester, and Philadelphia criteria were established from some of the largest and most applicable studies.16,18,19,21
Boston Protocol. Researchers in Boston prospectively tested the safety and efficacy of outpatient management of 504 febrile (> 38° C) infants (1-3 months old) with intramuscular (IM) ceftriaxone after meeting specific low-risk criteria.15,30 Their low-risk criteria included a well-appearing child, no immunization or antimicrobial in last 48 hours, no evidence of soft tissue infection, and reliable caretaker. Low-risk laboratory results included peripheral white blood cell (WBC) < 20,000/microL, urinalysis (UA) < 10/microL, cerebrospinal fluid (CSF) WBC < 10/microL and clear chest x-ray. With a more liberal WBC cutoff than other studies, 27 (5%) of this low-risk group had culture-proven bacterial infections including UTIs, bacteremia, and gastroenteritis.30,31 However, all infants recovered uneventfully and none had significant complications from their initial evaluation.
Philadelphia Protocol. Investigators in Philadelphia studied 747 infants 29-60 days of age with rectal temperature ≥ 38.2° C to evaluate the appropriateness of outpatient management in low-risk patients. Using a more conservative screening guideline, 287 (40%) of the 747 infants were deemed to be at low risk for bacterial disease. In contrast to the Boston group, low-risk patients were not treated empirically with antibiotics. The sensitivity and specificity of this screening tool for identifying patients with SBI was 98% and 42%, respectively.31 Only one child in the low-risk group had a bacterial disease, translating to a negative predictive value (NPV) of > 99%. Given the high negative predictive value that was reproduced in a follow-up study by the same group,20 the authors concluded that selected febrile infants (older than 28 days of age) can be managed as outpatients without antibiotics after a full septic workup, given that reliable follow-up care is arranged with a physician.31
Rochester Protocol. Another group of researchers aimed to identify low-risk febrile infants (rectal temperature ≥ 38° C) younger than 60 days.21 In their study group of 931 well-appearing infants, 437 (47%) qualified as low risk for bacterial infection. Only 5 low-risk infants had an SBI, yielding a negative predictive value of 98.9%. However, their sensitivity was relatively lower at 92.4%. Unlike the Boston and Philadelphia studies, they did not use a uniform sepsis evaluation on all their infants and empiric antibiotic treatment was inconsistent.31 Despite these limitations, there were no reported adverse outcomes, further supporting a less aggressive management strategy in low-risk infants.32
Limitations of Protocols
Although these protocols have been well-validated, they are not absolute. The Philadelphia and Rochester protocols use criteria that allow for a high sensitivity; thus, they can advocate sending low-risk patients home without empiric antibiotics. With a lower sensitivity and negative predictive value, the Boston protocol recommends a full septic workup, including lumbar puncture (LP), and empiric antibiotics for low-risk patients pending culture results.
Studies that have evaluated the applicability of the various screening strategies to infants younger than 28 days have found an increased number of missed SBI in this age group and higher prevalence of SBI when compared to their older counterparts.10-12,33,34 The patient population in the Boston and Philadelphia studies excluded infants under 1 month of age. A retrospective study that applied these low-risk criteria to febrile infants 1-18 days of age revealed a negative predictive value of 97% for both protocols.37 The authors concluded that these protocols, when applied to infants 28 days or younger, will miss 3% of febrile infants with SBI. The Philadelphia and Rochester protocols also were re-evaluated recently in a prospective study that enrolled infants 56 days or younger. The authors here found the negative predictive value for the Philadelphia and Rochester criteria to be 97.1% and 97.3%, respectively, compared to the original report of 99.7% and 98.9%, respectively.38 Adequate compensation for this small percentage of missed SBI is addressed through the admission of all infants less than 1 month of age and the establishment of timely follow-up.
American College of Emergency Physicians (ACEP). ACEP revised its 1993 pediatric fever policy in 2003. In a question-answer approach, it advises evidence-based recommendations that parallel those expressed by this article. Namely, ACEP advocates the admission of all febrile infants less than 1 month for observation and parenteral antibiotics. Furthermore, infants should not be viewed in discrete age-defined categories but rather as a continuum of disease, growth, and maturation.
Recognition and General Approach: Laboratory Studies and Diagnostic Radiology
WBC. Faced with a well-appearing infant with a fever, the patient should be risk stratified using developed protocols as described above. One of the simplest tests to obtain is a complete blood count (CBC). The magic number for WBC seems to be less than 15,000-20,000 to be considered low risk. In a recent study, Gomez and colleagues found that a WBC was not an accurate measure or predictor of SBI.39 Other studies have reproduced similar results.40,41 Thus, although a good parameter to monitor during treatment and perhaps identify certain infants who might be more at risk, the WBC should not be used as the only basis for decision making in obtaining blood cultures or pursuing further workup.
Blood Culture (BC). Blood culture is advocated as one of the initial tests to obtain in febrile infants. However, the reality is that this practice can vary among clinicians. Out of 1,125 infants admitted with FWS (fever without source), Gomez and colleague found that blood cultures were obtained in 1,018 (91.5%). Those which did not receive a blood culture were older infants; 80.4% were older than 2 months.39 Another large retrospective study found that non-pediatric ED physicians were more likely to obtain chest x-ray and lumbar puncture, yet less likely to obtain blood cultures or urine cultures.42 The authors reasoned this heterogeneity might be due to the general ED physician's lack of awareness regarding the sequelae of a missed bacteremia or UTI.
Despite the obvious heterogeneity in practice, which probably stems from a combination of experience and training, it is recommended to obtain blood cultures in all febrile infants. The culture can be obtained from the same site of IV placement after sterile preparation of the skin.
Recognition and General Approach: Inflammatory Markers
C-reactive Protein (CRP). CRP is an acute phase protein produced in the liver in response to inflammatory stress. Its level rises within 6 hours and peaks at 48 hours. Thus, CRP is a common tool utilized by the clinician to predict bacterial infection. Garcia found that at a cutoff point of 2 mg/dL, CRP performed at a sensitivity and specificity of 53% and 85%, respectively.41 Another larger study, also conducted in Spain, found a specificity of 93.8% but a sensitivity of only 69.6% with the cutoff point of 70 g/L (7 mg/dL).39
Erythrocyte Sedimentation Rate (ESR). Erythrocyte sedimentation rate is the rate at which red blood cells precipitate in 1 hour. This rate will increase if the balance between presedimentation factors (i.e., fibrinogen) and those factors resisting sedimentation is offset; as in acute inflammation due to an infectious process. In the same study by Garcia, at a cutoff of 20 mm/hr, ESR had a sensitivity and specificity of 38% and 75% respectively in predicting SBI in 0- to 90-day-old infants.41 In another retrospective study of 1,051 children aged 1 month to 10 years old, sedimentation rate > 30 mm was not a reliable predictor for occult bacteremia.44 Hiew et al examined the haematologic indices of 100 infants < 3 months old and concluded that an elevated sedimentation rate, when used in combination with raised CRP or leukocyte counts, was significantly associated with SBI.43 Erythrocyte sedimentation rate is at best an insensitive and nonspecific marker for bacterial infection. It may be useful in conjunction with other inflammatory markers and clinical information but should not be relied upon solely to dictate management.
Procalcitonin (PCT). Procalcitonin is produced by the parafollicular cells of the thyroid and the neuroendocrine cells of the lung and intestine. It serves as the precursor of the hormone calcitonin. Under physiological stress, the level of procalcitonin will rise accordingly. Although this lab value is not routinely obtained in U.S. EDs, there is evidence to support that PCT offers better specificity than CRP in detecting bacterial infections in infants. One study suggests that at a cutoff of 0.53 ng/mL, PCT had a sensitivity and specificity of 65.5% and 94.3% for detecting bacterial vs. viral infections.45 Another more recent study concluded that at optimal cutoff value of 0.6 ng/mL, PCT carried a sensitivity, specificity, and positive predictive value (PPV) of 100%, 65%, and 67%, respectively, for diagnosing nosocomial infection in neonates.46 Mantadakis et al conducted a meta-analysis of 10 studies with the conclusion that in children with culture-proven UTI, a serum PCT level > 0.5 ng/mL predicts reasonably well the presence of acute renal parenchymal involvement in DMSA scintigraphy.47 Children in the involved studies ranged from 1 week to 16 years old. Procalcitonin may be a useful tool in identifying children at risk for severe UTI with kidney involvement (i.e., pyelonephritis).
IT Ratio. Studies that looked at WBC found it to be an unreliable marker, both in sensitivity and specificity, for detecting SBI. As such, some researchers have explored the IT (immature-to-total neutrophil) ratio as perhaps a more convincing marker. In a small study, Janota et al found the IT ratio to be poorly correlated with neonatal sepsis in low birth weight infants.48 Looking at febrile children younger than 2 years, Kuppermann found that although children with SBI had a higher mean absolute neutrophil count than children with respiratory infections, there was no difference in band-neutrophil ratio between the two groups.49
Urinalysis (UA). Infants are unable to verbalize the clinical symptoms associated with a UTI. Fever can be, many times, the only presenting sign.
The practice guidelines advocated a urinalysis to be obtained in all febrile children without source under 3 months.1 The guideline used evidence in a report by Jaskiewicz et al which found that of the 227 low-risk infants under 28 days, one had an SBI: a urinary tract infection caused by E. coli.21 In the large prospective cohort study that led to the Boston protocol, UTIs were among the common causes of culture-positive bacterial diseases in the 503 infants ages < 3 months deemed to be low-risk for SBI.19 In another large study of 2,411 children (< 2 years) presenting with fever ≥ 38.5° C, researchers found a prevalence of 3.3% for UTI.50 Febrile children are more likely to have a UTI than an occult bacteremia; uncircumcised boys and Caucasian heritage in girls are risk factors.50
Urine Culture. Due to the high prevalence of UTIs in young infants, it is hard to argue against obtaining a urinalysis. The next question becomes is a urine culture necessary for all febrile infants? Hoberman et al looked at this question in their study of 4,253 children younger than 24 months evaluated in the ED.51 They examined the use of "enhanced urinalysis" to evaluate for pyuria (10 or more WBCs per HPF in an unspun urine specimen) and bacteriuria (any bacteria/10 oil immersion fields on a Gram-stained smear). The presence of both pyuria and bacteriuria defined a positive urinalysis. The sensitivity and PPV of enhanced UA for identifying positive urine culture was 84.5% and 93.1%, compared to 65.6% and 80.8% for the standard UA, respectively. The authors advocate always obtaining a urine culture in patients with previous UTI, urinary tract abnormality, and those who will be treated presumptively with antibiotics. In a meta-analysis of literature concerning rapid diagnostic tests for UTI, Gorelick and Shaw demonstrated that a urine gram stain had the optimal combination of sensitivity and false-positive rate for detecting UTI in children; 93% and 4% respectively.52 Furthermore, they found the urine dipstick to perform nearly as well with either a positive leukocyte esterase or nitrite test outcome, with sensitivity of 88% and false-positive rate of 4%. A urine culture should be ordered along with the urinalysis due to the inability of this age group to express symptoms of a UTI and the serious potential sequelae (missing underlying structural abnormalities of the urinary tract system) that may result from a delayed diagnosis.
Due to the unacceptable contamination rate associated with "bagged" urine samples, ED physicians should obtain a catheterized urine sample for urinalysis and urine culture. In addition to infants less than 3 months of age, urine culture is especially recommended in high-risk populations, which includes uncircumcised boys under 1 year of age, boys under 6 months of age, and girls under 24 months. Apprehensive parents should be reassured that the procedure is part of a thorough workup in the interest of the child.
Stool. The established protocols call for a stool sample if the clinician evaluating the infants deems it a useful test after obtaining the history and physical examination.49 Bacterial enteritis occurs in approximately 3% of febrile children.33 The presence of polymorphonuclear cells (> 5 PMNs/hpf) in the stool seems to be the best predictive variable for a positive bacterial stool culture with sensitivity and specificity of 85% and 88%, respectively.53 A history of abrupt onset of diarrhea, greater than 4 stools per day with absence of vomiting before diarrhea has a diagnostic sensitivity of 86% and specificity of 60%.53
Lumbar Puncture. Although there is almost no argument regarding a complete septic evaluation, including lumbar puncture, in febrile infants under 1 month, there is room for debate in older infants. The decision to perform a lumbar puncture on an older infant depends on the clinical exam of the patient, risk factors for SBI, comfort level of the physician, and reliability of follow-up. For instance, a clinician using the Boston protocol might choose to perform a lumbar puncture on a well-appearing 2-month-old and discharge the patient with antibiotics and close follow-up, pending CSF results. With the average incidence of meningitis in this group being 1.7%, the absence of lethargy, irritability, poor feeding, bulging fontanel, or vomiting can be reassuring (NPV = 98.8%); however, in isolation these clinical signs are poor predictors of positive CSF cultures.33,54
Barraff pointed out that in studies which looked at the cohort of febrile infants under 3 months who met low-risk criteria, those using lumbar puncture as part of the diagnostic evaluation had a combined total of 1,051 "low-risk" infants, of which 30 (2.9%) had an SBI.30 In a study assessing the utility of low-risk criteria, excluding a lumbar puncture, Jaskiewicz found only 5 out of 437 infants who met the low-risk criteria to have an SBI; none of which had meningitis.21 Therefore, some clinicians support the exclusion of the lumbar puncture in infants > 1 month of age given that they qualify as "low-risk" after clinical exam and diagnostic tests, with reliable follow-up and receptive parents. Furthermore, although the presence of UTI does not exclude other concurrent SBIs, Schadower et al found infants aged 29-60 days with cultured proven UTIs were at very low risk of adverse events after meeting certain low-risk criteria, including well-appearing and benign medical history.55 Only 1 child in the 1,206 low-risk infants had an adverse event, yielding a negative predictive value of 99.9%. The decision to perform a lumbar puncture in this age group should be up to the discretion of the treating physician with the understanding that omission of this test carries with it potential of greater risk.56 However, it always should be done if empirical antibiotics are to be given.30
Chest X-ray. The need to obtain chest radiograph in all febrile infants younger than 3 months remains controversial. There are studies to suggest the probability of a positive or abnormal chest x-ray finding to be 1%-3% in infants presenting without respiratory symptoms.30,57,58 Well-appearing children with abnormal chest x-ray are likely to have viral infections. Children with hyperpyrexia and marked leukocytosis (WBC > 20,000/mm3) are more likely to have occult bacterial pneumonia.59 Despite the low probability of a positive finding, many physicians order the chest x-ray in fear of missing an occult pneumonia; radiographs should be done only in infants who present with respiratory symptoms, abnormal pulse oximetry, hyperpyrexia, or marked leukocytosis.
Evaluation and Management: Neonates (0-28 days)
There is general consensus among physicians regarding the management of the febrile neonate. With a documented fever, the neonate (0-28 days of age) should undergo a sepsis evaluation and be admitted to the hospital for 48-72 hours of antibiotic coverage.8 Despite the relatively low risk of occult bacteremia in neonates who qualify as low risk, the recommendation continues to support a full septic diagnostic evaluation and admission due to the higher rate of occult bacteremia, lower immunocompetency, lack of reliable physical and verbal signs, and the suggested lower NPV of protocols when applied to this age group.10,18,21,49
Diagnostic evaluation in this age group should be comprehensive including CBC with differential, blood cultures, catheterized urinalysis and urine culture, CSF analysis, chest x-ray (if indicated), and stool studies (if indicated). A reasonable choice for empiric antibiotics includes ampicillin and an aminoglycoside or cefotaxime. If the infant is suspected to have meningitis, cefotaxime may be preferred. Ceftriaxone generally is avoided in this age group due to the theoretical possibility of biliary sludge. (See Figure 1.)
Figure 1. Algorithm for Management of Febrile Neonate

Evaluation and Management: Young Infants (29-60 days) Although there is little debate on the workup of febrile infants less than 1 month of age, there exist considerable variations for management of older infants. Established protocols, including the Rochester criteria, perform relatively well in identifying low-risk infants, with the Rochester criteria generally having an NPV of 97%-100%.21,33,60,61 This is especially true for infants older than 1 month.
Ill-appearing. Despite protocols and guidelines, ED physicians should not dismiss the history and clinical exam. An ill-appearing child trumps any "pretest probability" of harboring an SBI. The established protocols have conveniently provided guidelines to identify low-risk infants. However, it can be difficult to define what the "ill-appearing" infant looks like. Infants who have intractable vomiting, appear dehydrated, lethargic, inconsolable, failure to thrive, should be admitted to the hospital following a full septic evaluation and IV antibiotics. Infants with questionable follow-up or unreliable parents also should be admitted.
Well-appearing. Well-appearing infants in this age group are more challenging to manage. This stems from the technicality of the established guidelines. For instance, one might argue the arbitrariness of letting a 35-day-old infant go home vs. admitting a 28-day-old infant. Personal disposition of these patients will vary depending on the ED physician's training background and level of comfort with physical examination and diagnosis.42 Infants who meet the low-risk criteria by using any of the three established protocols can be safely managed as outpatients, granted that reliable follow-up is arranged.33,49,62,63 Antibiotics should not be provided to patients who are discharged home unless a lumbar puncture was performed (i.e., the Boston protocol approach) for concern of compromising later findings if the patient returns due to worsening condition. (See Figure 2.)
Figure 2. Algorithm for Infants 2-3 Months Old

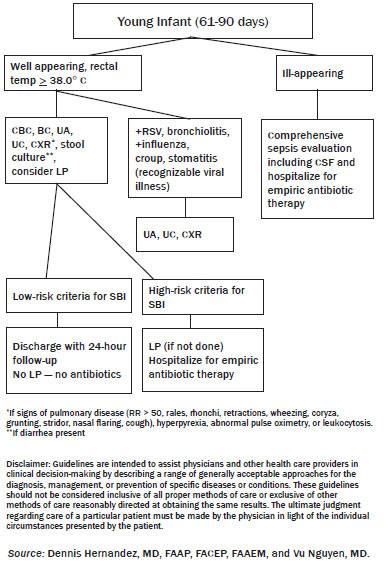
Evaluation and Management: Young Infants (61-90 days)
Ill-appearing. Although the disposition of febrile infants in this age group can warrant much debate, the presence of an ill-appearing infant rather simplifies management. A child who does not meet the low-risk protocol or is ill-appearing should receive a comprehensive septic evaluation in the ED and be admitted for observation and IV antibiotics.
Well-appearing, Immunized. With the introduction of the Hib vaccine in the late 1980s and more recently the pneumococcal vaccine, the incidence of SBI in children has decreased dramatically. One study in Northern California found that routine vaccination with pneumococcal vaccine resulted in an 84% reduction of S. pneumoniae bacteremia (1.3-0.2%) and a 67% reduction in overall bacteremia (1.6-0.7%) in the study population of children 3 months to 3 years old.64 Interestingly, during this 5-year study, there was a 35% reduction in total blood cultures drawn in outpatient pediatric clinics but it remained unchanged in emergency departments. ED physicians should be more conservative in their management, to not miss an occult bacteremia in a child with an incomplete immunization series (only 1 vaccine) without an established relationship with the family and guaranteed follow-up.
Parents should be instructed to return in 24 hours or follow up with their primary care physician the following day for re-evaluation. It would be prudent to call the patient's primary care physician to arrange this follow-up.
Well-appearing, Not Immunized. Whatever the reason might be (religious, poverty, negative media, birth in foreign country, etc.), ED physicians still face parents who bring in an unimmunized child for evaluation. These children benefit from a more mature immune system than younger infants but lack the added protection of their immunized counterparts. Unfortunately, it is more likely that these same patients lack the appropriate follow-up for outpatient management. Low-risk infants in this subset will benefit from admission for observation pending cultures from urine, blood, and spinal fluid (if the ED physician deems appropriate). (See Figure 3.)
Figure 3. Algorithm for Infants 2-3 Months Old
Antibiotic Therapy
Routine hospitalization with administration of antibiotics can result in unnecessary risk of iatrogenic complications and high costs.65,66 Febrile newborns up to 1 month of age mandate a comprehensive evaluation and empiric antibiotics. The relatively low, but real, risk of infection by Listeria sp. in this group requires the inclusion of ampicillin as part of the empiric regimen. Although thoughts on the second antibiotic can vary, gentamycin or cefotaxime are reasonable choices. In older infants, empiric therapy should begin with ampicillin and cefotaxime or ceftriaxone.67 Vancomycin can be employed if MRSA or S. pneumoniae resistant to penicillins and cephalosporins is suspected.
Acyclovir. In infants younger than 28 days, herpes simplex virus (HSV) meningoencephalitis should be suspected if CSF pleocytosis is present with a negative gram stain. Clinical presentation may include seizures, ill-appearing, cutaneous vesicles, and evidence of elevated transaminases (elevated LFTs) or coagulapathy. The physician should inquire specifically about the laboring process and maternal risks. Neonatal HSV disease most commonly presents in the first and second week of life. Acyclovir should be added to the empiric regimen if HSV infection is suspected.
Difficult Clinical Situations
Traumatic Lumbar Puncture. A traumatic lumbar puncture inducing blood in the CSF can occur on occasion, usually by puncturing the radicular vessels which accompany each nerve root.68 Nigrovic and colleagues have shown that risk factors for a traumatic lumbar puncture includes operator experience, excessive movement, advancing needle with stylet in place, and lack of adequate local anesthesia.69 The presence of a family member, although intimidating to the clinician, can help with calming the patient and has not been shown to affect success rate.70 Traumatic lumbar puncture can be minimized by avoiding the previously mentioned risk factors. Depth of needle insertion in children younger than 3 months is between 1-1.5 cm while weight-based formulas for determination of depth are available for older children.71,72 Contamination of CSF with traumatic blood can make interpretation of cell counts and differential difficult. In these cases, the child should be treated presumptively for meningitis if pleocytosis is present until gram stain and culture data are available.
In the event of a dry tap, in an infant < 1 month of age, ED physicians may consider initiating empiric antibiotics and have the admitting physician attempt an LP at a later time, though in some instances, and according to local/institutional practices, hospitalization and observation without antibiotics may be a reasonable approach after a discussion with the admitting physician. One can infer this approach by the results of the Rochester study.
Parents Refuse Lumbar Puncture. The seemingly boisterous ED environment can be anxiety-provoking for parents. Although they recognize something is wrong with their child, they may not appreciate the severity of the illness and the mortality and morbidity associated with delayed diagnosis and treatment. If confronted with resistance, the clinician must advocate for the child and educate the parents about the risks and possible outcomes of not performing certain diagnostic procedures. Although parents' wishes should be honored, the refusal of diagnostic and therapeutic care in a life-threatening illness is deemed to be medical neglect; in this case, the clinician should involve the appropriate authorities and treat the child accordingly. However, in the context of a "low-risk" infant > 1 month of age, this refusal can be reasonably upheld with subsequent admission of infant for observation.
Patient on Antibiotic. Prior or current antibiotic therapy can confound the physical examination and laboratory results in the infant's evaluation. In fact, no prior history of recent or current antibiotics is part of the low-risk history in the Rochester and Boston criteria. Pretreated children can have a significant change in their clinical features with less high-grade temperatures and more well-appearing.73 A lumbar puncture of a pretreated infant might reveal pleocytosis with a negative culture. A full course of parenteral antibiotic is necessary because this negative culture can be interpreted to be either partially treated bacterial meningitis or aseptic meningitis.30
Subjective Fever at Home. Many times the clinician will examine an afebrile infant brought in for evaluation because of a subjective fever at home. In the event of a well-appearing child, the clinician must make an expert judgment on the reliability of the parents and what diagnostic workup is appropriate. Parents' anxiety can result in overestimation of the child's clinical appearance and degree of compromise.74 One study found that tactile or subjective fever determination by parents had a sensitivity of 67%, specificity of 84%, PPV of 33%, and NPV of 95%.75 Callanan found a sensitivity and specificity of 91% and 79% for perceived fever and 83% and 86% for temporal artery detection, respectively.76 Thus, subjective fever is relatively unreliable and rectal thermometry remains the most accurate method. Some conservative clinicians may treat a subjective fever by a reliable parent as a documented fever with appropriate workup. However, it is not unreasonable to observe the child in the ED for a few hours for the development of fever before appropriate discharge and follow-up if the patient remains afebrile and well-appearing.
Documented Fever at Home. An afebrile infant in the ED, with a documented fever at home, should be treated as a febrile infant with the appropriate septic workup.62 In a retrospective study of 292 infants, of which 244 infants had documented fever at home, Bonadio found 224 (92%) of the 244 infants with reported fever to have fever on presentation or during the next 48 hours of admission.77 Thus, a reported fever at home via thermometer should be managed similarly as a fever in the ED.
Fever and Bundling. The relatively high ratio of body surface area to body mass in newborns makes them more vulnerable to changes in body temperature from environmental causes. In very young infants, physicians particularly should question parents about bundling. Bundling and ambient heat may raise core temperature in newborns to "febrile" range.78 Another study suggests that while bundling can cause significant rise in skin temperature, this does not hold true in rectal temperature.79 However, their study group included older infants. Despite the susceptibility of body temperature in very young infants (neonates) to bundling, given the significant risk of SBI in this age group, it would be wise to manage these infants with the recommended approach for febrile infants < 1 month of age; especially if a rectal temperature was recorded. Well-appearing, older infants may benefit from a careful examination and selective workup including measured rectal temperature in the ED after unnecessary clothing and garments have been removed.
Concurrent Viral Infection
Respiratory Syncytial Virus (RSV). RSV is a prevalent viral cause of bronchiolitis, especially from fall to spring. Patients typically are younger than the age of 2 and often present with upper respiratory infection symptoms, coupled with lower respiratory symptoms including wheezing and retractions. Children with documented RSV infection are found to be at lower risk for SBI compared to their RSV-negative counterparts.80 Despite the lower risk of SBI in RSV-infected infants, the prevalence for SBI as a result of UTI remains appreciable and should be sought after.81,82 Given the data, infants older than 1 month of age with confirmed RSV may benefit from a more selective workup with minimization of cost and time.
Bronchiolitis. Although RSV is a key player in bronchiolitis, it only accounts for 50%-80% of cases.83 Thus, the clinical presentation of bronchiolitis does not necessitate infection with RSV. In a prospective study following admitted infants aged 90 days or less, Bilavsky et al discovered SBI in 30 of the 312 (9.6%) infants without bronchiolitis and 3 of 136 (2.2%) with bronchiolitis; UTI being the predominant infection in the first group and the only infection in the second.84 Kuppermann et al reported similar numbers with 1.9% of children with bronchiolitis having SBI vs. 16.3% in children without bronchiolitis.85 Although there is low yield in performing a sepsis evaluation in infants 1-3 months of age with clinical bronchiolitis and fever,86 a urine culture is at least indicated in the older infants.
Influenza. Influenza, especially in the appropriate season, can influence a provider's management of febrile infants. Studies have shown that children with diagnosed viral infections are less likely than their counterparts to have SBIs. Influenza is no exception. In a retrospective study of 705 children, aged 0-36 months, Smitherman found a lower incidence of bacteremia, UTIs, radiographic evidence of pneumonia, or any SBI in febrile children with influenza A.87 Concentrating on the age group of 0-3 months, Mintegi and colleagues found similar results. The incidence of SBIs was significantly lower in the positive RIT (rapid influenza test) vs. the negative RIT; (3/133; 2.65%) vs. (47/268; 17.5%), respectively.88 The utility of rapid influenza testing for febrile infants may translate to significantly decreased time spent in the ED and unnecessary testing.89
Concurrent Findings
Otitis media. The prevalence and epidemiology of otitis media has changed in the last few decades. There seems to be an increasing incidence in young children and earlier onset of acute otitis media (AOM). One likely explanation is the high rate of daycare attendance.90 Thus, the ED clinician should be vigilant of this source of infection. However, the presence of otitis does not correlate or increase the risk of an associated serious bacterial infection.91 S. pneumoniae and H. influenzae remain the common pathogens and an isolated AOM in infants under 3 months does not place them at higher risk for serious bacterial infections.92,93 In the evaluation of 137 infants < 2 months of age with AOM, Turner et al reported no SBI in 37 children who were low risk outside of having AOM.93 Infants who had a SBI (mostly UTIs, no bacterial meningitis) were high risk when excluding the diagnosis of AOM. Thus, it may be reasonable to treat an infant > 1 month of age with AOM, who is otherwise low risk, the same as a low-risk child without AOM.
Pneumonia. Community-acquired pneumonia continues to be a prominent cause of serious bacterial infections in children. Pneumonia in neonates younger than 3 weeks usually is caused by an infection acquired from the mother at birth (i.e., E. coli, Group B streptococci, L. monocytogenes). Streptococcus pneumoniae and viruses are the most common culprits in older infants.94 Unfortunately, there has not been a particularly useful low-yield criterion developed to effectively identify febrile infants as low risk for pneumonia and thereby decrease the use of radiographs. An abnormal pulse oximetry reading, particularly less than 95%, has been shown to effect clinician's disposition of febrile infants and aid in diagnosing occult pneumonia.95,96 Bachur et al reported radiographic pneumonia in 26% of children with a temperature of 39.0° C or greater and a WBC count 20,000/mm3 or greater even though they displayed no clinical evidence of pneumonia.97 Recently, using the same high-risk parameters, but on a slightly younger patient group (under 3 years of age), Mintegi reports a lower incidence of 13.3% in those in which chest radiographs were obtained.98 The introduction of PCV7 may be a likely reason for this lower incidence. Although obtaining chest x-ray in all febrile infants is controversial, a chest x-ray should be obtained in infants with respiratory symptoms, abnormal pulse oximetry reading, hyperpyrexia, or marked leukocytosis.
Urinary Tract Infection. No discussion of febrile infant management is complete without addressing the high incidence of UTIs in these patients. The rate of urinary tract infection for premature infants (2.9%) surpasses that for full-term infants (0.7%)99 with a male predominance in the first 3 months; after which, the dominance shifts to females.100 In febrile infants younger than 2 months, UTI has a prevalence of 7.5%.101 In a practice parameter published in 1999, the American Academy of Pediatrics supported the cost-effectiveness of pursuing the diagnosis of UTI in girls less than 2 years of age and uncircumcised boys less than 1 year of age.102 The parameter states the diagnosis of UTI should not be made with a urine culture obtained by the bag method, especially in an ill-appearing child. The presence of a UTI does not exclude other serious bacterial infections. UTIs also repeatedly have been shown to be a significant source for SBI even in clinically or laboratory-confirmed viral infections.87-89
Soft-tissue infection. Aseptic delivery techniques have rendered omphalitis an uncommon complication. However, a thorough physical exam should be conducted to help exclude this diagnosis, particularly if the mother had a home delivery. Untreated omphalitis can progress to abdominal wall cellulitis and necrotizing fasciitis.103
Cellulitis in infants under 3 months of age usually is due to Group B streptococci. Infants in this age group should receive a comprehensive septic workup due to the associated risk with bacteremia.104
Joint infection. Although an uncommon cause of SBI, most cases of pyogenic arthritis occur in children under 3 years of age. S. aureus remains the most common cause of pyogenic arthritis in all age groups. Since introduction of the Hib vaccine, K. kingae has become the most common gram-negative arthritis in children aged 2 months to 5 years.105 Joint symptoms often are preceded by trauma or upper respiratory tract infection.106,107 Suspect the diagnosis with a febrile, irritable infant, especially with movement of the limbs and joints. Older children might complain of joint pain along with limping. Redness, swelling, and warmth might be appreciated over the affected joint. If suspected, blood and joint fluid should be sent for aerobic and anaerobic cultures. Joint fluid with a WBC count of 50,000/mm3 or greater is consistent with a bacterial infection.106 Suspicion of specific organism, including N. gonorrhea and S. pygogenes, should be pursued with appropriate testing. Management involves prompt decompression of the affected joint and appropriate antibiotic therapy. Infants younger than 2 months should receive nafcillin and a third-generation cephalosporin to cover S. aureus and enteric gram-negative bacteria.106,107 Ceftriaxone should be used if N. gonorrhea is suspected. This antibiotic regimen is also appropriate for osteomyleitis.
Diarrhea. As previously mentioned, the incidence of bacterial enteritis in febrile children is approximately 3%.33 Both the Philadelphia and Rochester protocols include stool studies to rule in low-risk patients if clinician deems appropriate. Stool samples should be obtained for cultures and WBC smear from infants with a history of vomiting, diarrhea, bloody or mucoid stools.
Current Challenges and Controversies
The management of febrile infants has been nothing short of controversial since the 1980s and likely will continue to be so. Success of the Hib and more recently, pneumococcal vaccine, has convinced many physicians that perhaps unnecessary tests are being performed on low-risk infants, specifically older infants. In the evaluation of 429 febrile infants 57-180 days old, Hsiao reported a SBI rate of 10.3%, most of which were UTI and no bacterial meningitis was diagnosed.108 No infants, aged 2-3 months, had a positive blood culture. This suggests that infants 2-3 months of age perhaps can be managed less conservatively and be grouped with their older counterparts. In a cost-effectiveness analysis, Lee et al demonstrated that if the rate of occult bacteremia falls below 0.5%, then an approach that uses empiric testing and treatment should be eliminated.109 Although this cutoff goal has been reached for children aged 6 months or older,110 the inclusion of younger infants remains controversial.
Pitfalls to Avoid
The ill-appearing infant, although unfortunate, can simplify things for the ED physician in terms of management. On the other hand, the well-appearing febrile infant requires a more tailored approach that can be intimidating for residents and young clinicians. Once the decision is made to discharge the patient from the emergency department, the ED physician should contact the primary pediatrician to ensure prompt following and continuity of care. A concise account of this conversation should be documented on the patient's chart. Furthermore, empiric antibiotics should not be given unless a full workup, specifically a lumbar puncture, has been performed. It falls on the ED physician to deem when it is inappropriate to send a low-risk febrile infant home (i.e., unreliable parents, poor follow-up, out-of-town patient). Be wary of infants with a history of prematurity and congenital disease; despite a benign exam, these infants are no longer "low risk" per the established protocols.
Summary
Much has been written on the topic of febrile infants over the past few decades. A febrile infant continues to be anxiety-provoking for parents and physicians alike. However, using scientific data, expert opinion, and personal experience, the wise clinician will formulate the best approach for each patient.
No established protocols or pre-test probability should trump a good history and physical examination. An ill-appearing infant, regardless of age, will benefit from a full septic workup, parenteral antibiotics, and admission with observation pending culture results. Infants with a history of prematurity or congenital disease are not considered "low risk" and should be evaluated with a high suspicion for SBI. Fever, although an important parameter should not be the only factor to determine management. An afebrile infant who appears clinically concerning should be managed appropriately. On the contrary, a well-appearing afebrile child with a documented fever at home should be treated like a febrile child in the ED.
Given the inherent risk of SBI and the unacceptable performance of established protocols when applied to infants < 1 month of age, the recommendation remains to perform a comprehensive evaluation on all febrile infants in this age group including hospital admission and parenteral antibiotics. Ampicillin and aminoglycosides are good antibiotic choices. Cefotaxime should replace the aminoglycoside if meningitis is suspected. It is extremely important not to forget about HSV in these young infants. If HSV infection is considered, a dose of acyclovir should be given pending PCR results.
Well-appearing older infants, aged 1-2 months, can be managed through the application of low-risk criteria. Although significant variability exists in the workup of these patients, at the very least a CBC with differential, blood cultures, catheterized urinalysis, and urine culture are recommended. A chest x-ray may be useful with a history of respiratory symptoms, hyperpyrexia, leukocytosis, or abnormal pulse oximetry. Antibiotics should not be given if a lumbar puncture is not performed. Reliable outpatient follow-up is a reasonable option in those infants who meet the low-risk criteria. Trustworthy parents should be instructed to bring the child to primary care physician's office for evaluation or back to the ED if that is not possible.
The introduction of the Hib and pneumococcal vaccine has led to a dramatic decrease in the rate of SBI in febrile children. Immunized infants older than 2 months have similar prevalence of SBI compared to their older counterparts and can be managed as such. These infants may perhaps benefit from a more personalized approach in workup to avoid unnecessary testing and hospitalization.
The evaluation and management of infants > 1 month of age also can be affected by the presence of concurrent viral infections. Infants with viral infections are at decreased risk of harboring SBI. Therefore a more limited workup could be entertained. However, UTIs continue to be a significant source of bacterial infection in these patients and so still must be pursued.
As more data become available concerning the true incidence of SBI in febrile infants in the post-pneumococcal vaccine era, along with the availability of rapid viral and bacterial testing in the ED, this dynamic area of medicine surely will continue to evolve. However, no protocols or testing should replace an astute clinician's history taking and physical examination coupled with evidence-based medicine to formulate the best management plan for each patient.
References
1. Baraff LJ, Bass G, Fleisher J, et al. Practice guideline for the management of infants and children 0 to 36 months of age with fever without source. Ann Emerg Med 1993;22:1198-1210.
2. Smitherman H, Macias C. Evaluation and management of fever in the neonate and young infant (less than three months of age). UpToDate Inc. Sept. 2010. Available at: www.uptodate.com/patients/content/topic.do?topicKey=~CqqEb58x8pe8BO. Accessed Dec. 8, 2010.
3. Baker MD, Avner JR, Bell LM. Failure of infant observation scales in detecting serious illness in febrile, four to eight week old infants. Pediatrics 1990;64:1040-1043.
4. Barren JM, Rothrock SG, Brennan JA, Brown L. Pediatric Emergency Medicine. Philadelphia: Saunders/Elsevier. 2008: 291-298.
5. Smitherman H, Macias C. Definition and etiology of fever in neonates and infants (less than three months of age). UpToDate Inc. Sept. 2010. Available at: www.uptodate.com/patients/content/topic.do?topicKey=~dvyWqDEgEC2EMW. Accessed Dec. 8, 2010.
6. Marom R, Sakran W, Antonelli J, et al. Quick identification of febrile neonates with low risk for serious bacterial infection: An observational study. Arch Dis Child Fetal Neonatal ED 2007;92:15-18.
7. Rudd P. Is there a place for "drive thru" management of neonatal fever? Not yet! Arch Disease Childhood Fetal Neonatal 2007;92:2-3.
8. Strange GR. Pediatric Emergency Medicine. New York: McGraw Hill Medical. 2009; 9-12.
9. Baskin MN. The prevalence of serious bacterial infections by age in febrile infants during the first 3 months. Pediatr Ann 1993;22:462.
10. Avner J, BakerM. Management of fever in infants and children. Emerg Med Clinics N A 2002;20:49-67.
11. Alpern ER, Alessandrini EA, Bell LM, et al. Occult bacteremia from a pediatric emergency department: Current prevalence, time to detection, and outcome. Pediatrics 2000;106: 505-511.
12. Lee GM, Harper MB. Risk of bacteremia for febrile young children in the post-Haemophilus influenzae type b era. Arch Pediatr Adolesc Med 1998;152:624-628.
13. Kaplan SL, Mason EO, Wald ER, et al. Decrease of invasive pneumococcal infections in children among 8 children's hospitals in the United States after the introduction of the 7-valent pneumococcal conjugate vaccine. Pediatrics 2004;113:443-449.
14. Black S, Shinefield H, Fireman B, et al. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Northern California Kaiser Permanente Vaccine Study Center Group. Pediatr Infect Dis J 2000;19:187-195.
15. Black S, Shinefield H, Fireman B, et al. Postlicensure surveillance for pneumococcal invasive disease after use of heptavalent pneumococcal conjugate vaccine in Northern California Kaiser Permanente. Pediatr Infect Dis J 2004;23:485-489.
16. Dagan R, Sofer S, Phillip M, Shachak E. Ambulatory care of febrile infants younger than 2 months of age classified as being at low risk for having serious bacterial infections. J Pediatrics 1998;112: 355-360.
17. Anbar R, Richardsondecorral V, Omalley P. Difficulties in universal application of criteria identifying infants at low risk for serious bacterial infection. J Pediatrics 1986;109:483-485.
18. Baker MD, Bell LM, Avner JR. Outpatient management without antibiotics of fever in selected infants. N Engl J Med 1993;329:1437.
19. Baskin M, Orourke E, Fleisher G. Outpatient treatment of febrile infants 28 to 89 days of age with intramuscular administration of ceftriaxone. J Pediatrics 1992;120:22-27.
20. Baker MD, Bell LM, Avner JR. The efficacy of routine outpatient management without antibiotics of fever in selected infants. Pediatrics 1999;103:627-631.
21. Jaskiewicz JA, McCarthy CA, Richardson AC, et al. Febrile infants at low risk for serious bacterial infectionAn appraisal of the Rochester criteria and implications for management. Febrile Infant Collaborative Study Group. Pediatrics 1994;94:390.
22. Dagan R, Powell KR, Hall CB, Menegus MA. Identification of infants likely to have serious bacterial infection although hospitalized for suspected sepsis. J Pediatr 1985;107:855.
23. Broner CW, Polk SA, Sherman JM. Febrile infants less than eight weeks old: Predictors of infection. Clinical Pediatrics 1990;29:438-443.
24. Chiu CH, Lin TY, Bullard MJ. Identification of febrile neonates unlikely to have bacterial infections. Pediatr Infect Dis J 1997;16:59-63.
25. Crain EF, Gershel JC. Which febrile infants younger than two weeks of age are likely to have sepsis? A pilot study. Pediatr Infect Dis J 1988;7:561.
26. Huppier AR, Eickhoff JC, Wald ER. Performance of low-risk criteria in the evaluation of young infants with fever: Review of the literature. Pediatrics 2010;125:228.
27. Goldman RD, Scolnik D, Chauvin-Kimoff L, et al. Practice variations in the treatment of febrile infants among pediatric emergency physicians. Pediatrics 2009;124:439-445.
28. Bergman DA. Does clinical presentation explain practice variability in the treatment of febrile infants? Pediatrics 2006;117:787-795.
29. Pantell RH. Management and outcomes of care of fever in early infancy. JAMA 2004;291:1203-1212.
30. Baraff L. Management of fever without source in infants and children. Ann Emerg Med 2000;36:602-614.
31. Smitherman HF, Macias CG. Strategies for the evaluation of fever in neonates and infants (less than 3 months). UpToDate 2010. Sept. 2010. Available at: www.uptodate.com/patients/content/topic.do?topicKey=~CqqEb58x8pe8BO. Accessed Dec. 8, 2010.
32. Mccarthy CA, Powell KR, Jaskiewicz JA, et al. Outpatient management of selected infants younger than two months of age evaluated for possible sepsis. Pediatr Infect Dis J 1990;9:385-388.
33. Slater M, Krug S. Evaluation of the infant with fever without source: An evidence based approach. Emerg Med Clin N A 1999;17:97-126.
34. Ishimine P. Fever without source in children 0 to 36 months of age. Pediatric Clin N A 2006;53:167-194.
35. Baker MD, Bell LM. Unpredictability of serious bacterial illness in febrile infants from birth to 1 month of age. Arch Pediatr Adolesc Med 1999;153:508-511.
36. Ferrera P. Neonatal fever: Utility of the Rochester criteria in determining low risk for serious bacterial infections. Am J Emerg Med 1997;15:299-302.
37. Kadish HA, Loveridget B, Tobeyt J, et al. Applying outpatient protocols in febrile infants 1-28 days of age: Can the threshold be lowered? Clin Pediatr 2000;39: 81-88.
38. Garra G, Cunningham SJ, Crain EF. Reappraisal of criteria used to predict serious bacterial illness in febrile infants less than 8 weeks of age. Acad Emerg Med 2005;12:921-925.
39. Gómez B, Mintegi S, Benito J, et al. Blood culture and bacteremia predictors in infants less than three months of age with fever without source. Pediatr Infect Dis J 2010;29:43-47.
40. Bonsu B. Identifying febrile young infants with bacteremia: Is the peripheral white blood cell count an accurate screen? Ann Emerg Med 2003;42:216-225.
41. Cuello-Garcia CA, Gomez L, Ceballos J. Total white blood cell count, erythrosedimentation rate and C-reactive protein for the detection of serious bacterial infections in 0- to 90-day-old infants with fever without a source. Ann Pediatr 2008;68:103-109
42. Isaacman DJ, Kaminer k, Veligeti H, et al. Comparative practice patterns of emergency medicine physicians and pediatric emergency medicine physicians managing fever in young children. Pediatrics 2001;108:354-358.
43. Hiew TM, Tan AM, Cheng AK. Clinical features and haematological indices of bacterial infections in young infants. Singapore Med 1992;33: 124-130.
44. Berezin EN. Evaluation of the incidence of occult bacteremia among children with fever of unknown origin. Braz J Infect Dis 2006;10:396-399.
45. Lopez AF, Luaces Cubells C, Garcia JJ, Fernandez J. Procalcitonin in pediatric emergency departments for the early diagnosis of invasive bacterial infections in febrile infants: Results of a multicenter study and utility of a rapid qualitative test for this marker. Pediatr Infect Dis J 2003;22:895-904.
46. Jacquot A, Labaune J-M, Baum T-P, et al. Rapid quantitative procalcitonin measurement to diagnose nosocomial infections in newborn infants. Arch Dis Childhood Fetal Neonatal 2009;94:345-348.
47. Mantadakis E, Plessa E, Vouloumanou EK, et al. Serum procalcitonin for prediction of renal parenchymal involvement in children with urinary tract infections: A meta-analysis of prospective clinical studies. J Pediatr 2009;155:875-881.
48. Janota J, Stranak Z, Belohlavkova S. Interleukin-6, procalcitonin, C-reactive protein and the immature to total neutrophil ratio (I/T) in the diagnosis of early-onset sepsis in low birth weight neonates. Ceska Gynekol 2000;65:29-33.
49. Kuppermann N. Immature neutrophils in the blood smears of young febrile children. Arch Pediatr Adolesc Med 1999;153:261-266.
50. Shaw KN, Gorelick M, McGowan KL, et al. Prevalence of urinary tract infection in febrile young children in the emergency department. Pediatrics 1998;102: E16.
51. Hoberman A, Wald E. Urinary tract infections in young febrile children. Pediatr Infect Dis J 1997;16:11-17.
52. Gorelick MH, Shaw KN. Screening tests for urinary tract infection in children: A meta-analysis. Pediatrics 1999;104:E54.
53. DeWitt T, Humphry K, McCarthy P. Clinical predictors of acute bacterial diarrhea in young children. Pediatrics 1985;76:551-556.
54. Rosenberg NM, Bobowski T. Clinical indicators for lumbar puncture. Pediatric Emerge Care 1988;4:5-8.
55. Schnadower D, Kuppermann N, Macias CG, et al. Febrile infants with urinary tract infections at very low risk for adverse events and bacteremia. Pediatrics 2010;126:1074-1083.
56. Baker MD, Avner JR. The febrile infant: What's new? Clin Pediatr Emerg Med 2008;9.4: 213-220.
57. Crain EF, Bulas D, Bijur PE. Is a chest radiograph necessary in the evaluation of every febrile infant less than 8 weeks of age? Pediatrics 1991;88:821-824.
58. Heulitt MJ, Ablow RC, Santos CC, et al. Febrile infants less than 3 months old: Value of chest radiography. Radiology 1988;167:135-137.
59. Bachur R, Perry H, Harper M. Occult pneumonias: Empiric chest radiographs in febrile children with leukocytosis. Ann Emerg Med 1999;33:166-173.
60. Pantell RH, Bernzweig J, Newman TB, et al. A new clinical prediction model for serious bacterial illness in febrile infants. AAP abstract. 1998.
61. Mccarthy CA, Powell KR, Jaskiewicz JA, et al. Outpatient management of selected infants younger than two months of age evaluated for possible sepsis. Pediatr Infect Dis J 1990;9:385-389.
62. Claudius I, Baraff LJ. Pediatric emergencies associated with fever. Emerg Med Clin North Am 2010;28:67-84.
63. Baraff LJ. Management of infants and young children with fever without source. Pediatr Ann 2008;37:673-679.
64. Klinkhammer M, Colletti J. Pediatric myth: Fever and petechiae. CJEM 2008;10:479-482.
65. Ogborn CJ, Soulen JL, DeAngelis C. Hospitalization vs outpatient treatment of young febrile infants: 10-year comparision. Arch Pediatr Adolesc Med 1995;149:94-97.
66. DeAngelis C, Joffe A, Wilson M, Willis E. Iatrogenic risks and financial costs of hospitalizing febrile infants. Am J Dis Child 1983;137:1146-1149.
67. Baker M. Evaluation and management of infants with fever. Pediatr Clin N A 1999;46: 1061-1072.
68. Evans R. Complications of lumbar puncture. Neurologic Clinics 1998;16:83-105.
69. Nigrovic L, Kuppermann N, Neuman M. Risk factors for traumatic or unsuccessful lumbar punctures in children. Ann Emerg Med 2007;49:762-771.
70. Nigrovic LE, McQueen AA, Neuman MI. Lumbar puncture success rate is not influenced by family-member presence. Pediatrics 2007;120:E777-E782.
71. Chong SY, Chong LA, Ariffin H. Accurate prediction of the needle depth required for successful lumbar puncture. Am J Emerg Med 2010;28:603-606.
72. Bilic E, Dadic M, et al. Calculating lumbar puncture depth in children. Coll Antropol 2003;27:623-626.
73. Rothrock SS, Green J, Wren D, et al. Pediatric bacterial meningitis: Is prior antibiotic therapy associated with an altered clinical presentation? Ann Emerg Med 1992;21.2:146-152.
74. Bonadio W. The history and physical assessments of the febrile infant. Pediatric Clin N A 1998;45:65-77.
75. Katz-Sidlow RJ, Rowberry JP, Ho M. Fever determination in young infants. Pediatr Emerg Care 2009;25: 12-14.
76. Callanan D. Detecting fever in young infants: Reliability of perceived, pacifier, and temporal artery temperatures in infants younger than 3 months of age. Pediatr Emerg Care 2003;19: 240-243.
77. Bonadio WA, Hegenbarth M, Zachariason M. Correlating reported fever in young infants with subsequent temperature patterns and rate of serious bacterial infections. Pediatr Infect Dis J 1990;9.3:158-160.
78. Cheng TL, Partridge JC. Effect of bundling and high environmental temperature on neonatal body temperature. Pediatrics 1993;92:238-240.
79. Grover G, Berkowitz CD, Lewis RF. The effects of bundling on infant temperatures. Pediatrics 1994;94:69-73.
80. Titus MO, Wright SW. Prevalence of serious bacterial infections in febrile infants with respiratory syncytial virus infection. Pediatrics 2003;112:282-284.
81. Levine DA, Platt SL, Dayan PS, et al. Risk of serious bacterial infection in young febrile infants with respiratory syncytial virus infections. Pediatrics 2004;113:1728-1734.
82. Oray-Schrom P, Phoenix C, St. Martin D, Amoateng-Adjepong Y. Sepsis workup in febrile infants 0-90 days of age with respiratory syncytial virus infection. Pediatric Emerg Care 2003;19:314-319.
83. Feigin RD, Welliver RC. 25: Bronchiolitis and Infectious Asthma. Textbook of Pediatric Infectious Diseases. Philadelphia: Saunders; 1987.
84. Bilavsky E, Shouval DS, Yarden-Bilavsky H,. A prospective study of the risk for serious bacterial infections in hospitalized febrile infants with or without bronchiolitis. Pediatr Infect Dis J 2008;27: 269-270.
85. Kuppermann N, Bank DE, Walton EA, et al. Risks for bacteremia and urinary tract infections in young febrile children with bronchiolitis. Arch Pediatr Adolesc Med 1997;151:1207-1214.
86. Melendez E, Harper MB. Utility of sepsis evaluation in infants 90 days of age or younger with fever and clinical bronchiolitis. Pediatr Infect Dis J 2003;22: 1053-1056.
87. Smitherman HF. Retrospective review of serious bacterial infections in infants who are 0 to 36 months of age and have influenza A infection. Pediatrics 2005;115:710-718.
88. Mintegi S, Garcia-Garcia J-J, Benito J, et al. Rapid influenza test in young febrile infants for the identification of low-risk patients. Pediatr Infect Dis J 2009;28:1026-1028.
89. Abanses JC, Dowd MD, Simon SD, Sharma V. Impact of rapid influenza testing at triage on management of febrile infants and young children. Pediatr Emerg Care 2006;22:145-149.
90. Pelton SI. Acute otitis media in an era of increasing antimicrobial resistance and universal administration of pneumococcal conjugate vaccine. Pediatr Infect Dis J 2002;21:599-604.
91. Nozicka CA, Hanly JG, Beste DJ, et al. Otitis media in infants aged 0-8 weeks. Pediatr Emerg Care 1999;15: 252-254.
92. Sakran W, Makary H, Colodner R, et al. Acute otitis media in infants less than three months of age: Clinical presentation, etiology and concomitant diseases. Int J Pediatr Otorhinolaryngology 2006;70:613-617.
93. Turner D, Leibovitz E, Aran A, et al. Acute otitis media in infants younger than two months of age: Microbiology, clinical presentation and therapeutic approach. Pediatr Infect Dis J 2002;21:669-674.
94. Ostapchuk M, Roberts D, Haddy R. Community-acquired pneumonia in infants and children. Am Fam Physician 2004;70: 899-908.
95. Mower WR, Sachs C, Nicklin EL, Baraff LJ. Pulse oximetry as a fifth pediatric vital sign. Pediatrics 1997;99.5:681-686.
96. Mower WR, Sachs C, Nicklin EL, et al. Effect of routine emergency department triage pulse oximetry screening on medical management. Chest 1995;108.5: 1297-1302.
97. Bachur R, Perry H, Harper M. Occult pneumonias: Empiric chest radiographs in febrile children with leukocytosis. Ann Emerg Med 1999;33:166-173.
98. Mintegi S, Benito J, et al. Occult pneumonia in infants with high fever without source: A prospective multicenter study. Pediatr Emerg Care 2010;26:470-474.
99. Edelmann CM, Orwo JE, Fine BP, et al. The prevalence of bacteriuria in full-term and premature newborn infants. J Pediatr 1973;82.1:125-132.
100. Ginsburg C, McCracken G. Urinary tract infections in young infants. Pediatrics 1982;69:409-412.
101. Crain EF, Gershel JC. Urinary tract infections in febrile infants younger than 8 weeks of age. Pediatrics 1990;86: 363-367.
102. Bergman D, Baltz R, Cooley J. Practice parameter: The diagnosis, treatment, and evaluation of the initial urinary tract infection in febrile infants and young children. Pediatrics 1999;103:843-852
103. Sawardekar KP. Changing spectrum of neonatal omphalitis. Pediatr Infect Dis J 2004;23.1:22-26.
104. Mittal MK, Shah SS, Friedlaender EY. Group B streptococcal cellulitis in infancy. Pediatr Emerg Care 2007;23.5:324-325.
105. Yagupsky P. From medical rarity to an emerging paediatric pathogen. Lancet Infect Dis 2004;4.6:358-367.
106. Gutierrez K. Bone and joint infections in children. Pediatr Clin North Am 2005;52:779-794.
107. Sonnen GM, Henry NK. Pediatric bone and joint infections. Diagnosis and antimicrobial management. Pediatr Clin North Am 1996;43:933-947.
108. Hsiao AL. Incidence and predictors of serious bacterial infections among 57- to 180-day-old infants. Pediatrics 2006;117.5: 1695-1701.
109. Lee GM, Fleisher GR, Harper MB. Management of febrile children in the age of the conjugate pneumococcal vaccine: A cost-effectiveness analysis. Pediatrics 2001;108.4:835-844.
110. Avner JR, Baker MD. Occult bacteremia in the post-pneumococcal conjugate vaccine era: does the blood culture stop here? Acad Emerg Med 2009;16:258-260.
Fever has been recognized as a symptom of illness for centuries. It is especially concerning when it is associated with a young infant.Subscribe Now for Access
You have reached your article limit for the month. We hope you found our articles both enjoyable and insightful. For information on new subscriptions, product trials, alternative billing arrangements or group and site discounts please call 800-688-2421. We look forward to having you as a long-term member of the Relias Media community.
