Rotavirus Infections in Children
Author: Maya C. Myslenski, MD, FAAP, Case Western Reserve University, MetroHealth Medical Center, Department of Emergency Medicine, Cleveland, OH.
Peer Reviewers: Larry B. Mellick, MD, MS, FAAP, FACEP, Professor, Department of Emergency Medicine and Pediatrics, Residency Program Director, Department of Emergency Medicine, Medical College of Georgia, Augusta; and Ann M. Dietrich, MD, FAAP, FACEP, Professor of Pediatrics, Department of Emergency Medicine, Children's Hospital, Columbus, OH.
Winter brings many challenges to the emergency department—RSV, influenza, and rotavirus. Rotavirus causes many deaths worldwide, but fortunately only a few deaths in developed countries. The toll in the United States, however, is measured in dollars—dollars spent on medical care and lost wages of young families caring for their ill infants and toddlers. This paper is part of our annual update on seasonal illness to better prepare you with the latest information to care for the patients you will encounter in the coming months. It is also relevant for those of you participating in the American Board of Emergency Medicine Lifelong Learning Self-Assessment (LLSA) program. The 2006 content of the LLSA includes acute infectious diarrhea. Our commitment to you is to keep you abreast of the essential information for your practice and to assist you in preparing for the recertification process.
—Sandra M. Schneider, MD, FACEP, Editor
Epidemiology
In North America, rotavirus gastroenteritis is a predictable winter epidemic every year—young children presenting to emergency departments and outpatient clinics with fever, vomiting, diarrhea, and varying degrees of dehydration. During the winter, the more severe an acute episode of gastroenteritis, the more likely it is to be due to rotavirus. Rotavirus is the most common cause of severe gastroenteritis among children less than 5 years of age. In the United States, rotavirus is responsible for 30-60% of severe diarrhea illnesses, 600,000 physician visits, 50,000 hospitalizations, 20-40 deaths, and a cost in excess of $1 billion each year.1 Worldwide, there are approximately 440,000 deaths, 2 million hospitalizations, 25 million outpatient visits including emergency department visits, and 111 million reported episodes of rotavirus infections every year.2
Rotavirus gastroenteritis is primarily a disease of infants and young children, with a peak incidence at 6-24 months of age, while occurrence is earlier in developing countries. By age 4, virtually all children have serologic evidence of prior rotavirus infection. Adults can be infected, but usually have asymptomatic or mild disease.3 Clinical illness is rare in neonates and uncommon in infants younger than 3 months because of protection by transplacental maternal antibodies, age dependent changes in the intestinal mucosa, and possibly breast milk. The premature infant who has not acquired maternal antibodies is susceptible to rotavirus gastroenteritis.4 Twenty-five percent of severe disease cases occur in children older than 2 years. However, a majority of children have cumulative immunity created by repeated infections by age 2. Most children are infected with rotavirus more than once. Initial infections usually are the most severe while subsequent infections are milder and may even be asymptomatic. After a single infection, 40% of children are protected against any subsequent infection with rotavirus, 75% are protected against diarrhea caused by subsequent rotavirus infection, and 88% are protected against severe rotavirus diarrhea. Subsequent infections offer progressively greater protection. No child with two previous infections has been documented developing subsequent severe rotavirus diarrhea.5
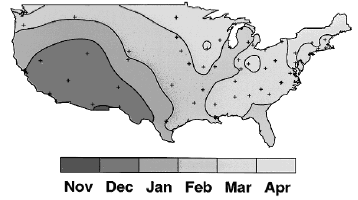
In temperate climates, rotavirus infections are most common during the cooler months of November through May. In the United States it spreads from west to east. The epidemic starts in Mexico and the southwestern United States in late fall, spreads rapidly in a northeastern direction, and ends in the northeastern states in the spring. (See Figure 1.) In tropical climates, rotavirus disease is endemic throughout the year, with some clustering in the cooler, drier months.
| Figure 1. The Rotavirus Epidemic Travels West to East Across the United States |
|
| Reprinted from: CDC. Laboratory based surveillance for rotavirus—United States, July 1996-June 1997. MMWR 46(46)1092-1094. |
Rotavirus is transmitted from one person to another primarily by the fecal-oral route, although respiratory transmission also has been suggested. Rotavirus gastroenteritis is highly communicable. Only 10-100 infectious virus particles are needed to cause infection.6 Large numbers of viruses are shed in the stool, from 100 to 1000 viruses per milliliter during acute illness. Shedding lasts on average for 7 days after onset of symptoms, but may persist as long as 3 weeks and may start before symptoms develop. Rotavirus survives on surfaces including tables, telephones, toys, and drinking fountains for long periods of time and spread of infection within families and in day-care centers is very common.7,8
Biology of the Rotavirus
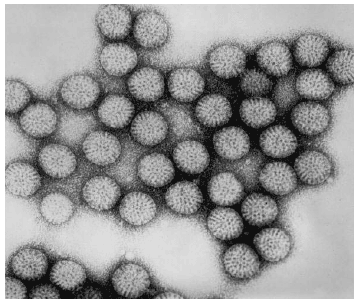
Rotavirus was first described as a causative agent of gastroenteritis in humans in 1973 when it was viewed in electron micrographs of duodenal mucosal biopsies from children with acute gastroenteritis. The virus is a wheel-like (see Figure 2), double-shelled icosahedron containing 11 segments of double-stranded RNA. There are at least seven distinct groups of rotaviruses labeled A through G. Only groups A, B and C are known to infect humans. Group A rotavirus is the most important cause of severe acute gastroenteritis in infants and young children worldwide. It contains two very important structural proteins within the outer capsid: VP4 antigen determines P serotype and VP7 determines G serotype. They stimulate production of neutralizing antibodies and are critical for vaccine development. The inner capsid contains the VP6 protein, which is the major rotavirus group antigen.
| Figure 2. The Distinctive Wheel-Shaped Rotavirus on Electron Microscopy |
|
| Source: CDC/Dr. Erskine Palmer. Public Health Illustration Library http://phil.cdc.gov/phil/home.asp |
Pathophysiology
Rotavirus causes disease by invading and destroying mature absorptive intestinal villus cells, resulting in loss of absorptive surface in the small intestine.9 Absorption of salt and water decreases, leading to net fluid secretion and osmotic diarrhea. Rotavirus infection of the enterocytes destroys the disaccharidases, which causes carbohydrate malabsorption and contributes to osmotic diarrhea also. Other factors may play a role in causing diarrhea as well, such as activation of the enteric nervous system (ENS) by rotavirus. Intestinal inflammation is accompanied by an increased tissue concentration of a large number of biologically highly active compounds that alone or together may activate enteric neurons, resulting in intestinal fluid and electrolyte secretion.10 Also, a nonstructural protein, NSP4, the rotavirus-associated enterotoxin, may trigger the release of amines and peptides from the endocrine cells of the gut to stimulate dendrites of free nerve endings located underneath the epithelial layer, thereby activating secretory nervous reflexes.10,11 Osmotic diarrhea can lead to dehydration and metabolic acidosis.
Clinical Features
The classic triad of symptoms includes fever, vomiting, and diarrhea, after an incubation period of 2-4 days. Typically there is an abrupt onset of fever and vomiting followed in 24-48 hours by watery diarrhea.12 Fever occurs in 50% of infected children and usually is low grade. Some children have fever over 102°F, which may trigger febrile seizures. Eighty to ninety percent of patients develop vomiting, which is non-bilious and can be frequent and intense, limiting attempts at oral hydration. Vomiting usually is brief, lasting 24 hours or less. Diarrhea begins later and typically is watery, without blood or mucus. There may be as many as 10-20 bowel movements a day, or in some cases, none at all. Approximately 10% of children hospitalized for rotavirus infection have only fever and/or vomiting at the time of admission.12 Symptoms usually last 3-7 days, but persistent diarrhea may be seen for many weeks.13
Laboratory Findings
Rotavirus gastroenteritis is a clinical diagnosis. Acute diarrhea in a young child, occurring during winter and associated with vomiting and a low-grade fever most likely represents rotavirus infection. Enzyme immunoassay and latex agglutination assays for group A rotavirus antigen detection in stool are available. It may be helpful in distinguishing rotavirus gastroenteritis from a bacterial process and can be used for confirmation of rotavirus infection. They offer about 90% specificity and sensitivity. If tested, stools of patients with rotavirus gastroenteritis typically do not contain white or red blood cells, however stool pH less than 6 and presence of reducing substances is common. Stool cultures should be performed in febrile children with frankly bloody diarrhea, or with prolonged diarrhea, but usually are not needed in an acute watery diarrhea of brief duration in otherwise healthy child. Complete blood cell count, serum electrolytes, urinalysis, urine and blood cultures usually are unnecessary. However, they are important when underlying diagnosis is unclear or there is a concern about sepsis, urinary tract infection, or a diagnosis other than acute gastroenteritis. Newborns and infants with urinary tract infections may have vomiting or diarrhea.14-17 A urine culture should be considered in febrile children 12 months of age and younger. In cases of severe dehydration or prolonged diarrhea and vomiting, electrolyte levels should be obtained to monitor rehydration. Children who are more severely volume depleted are at greater risk for electrolyte and acid-base abnormalities. Children with diarrhea lose bicarbonate in the stool, which may result in metabolic acidosis. Significant vomiting produces metabolic alkalosis. Although assessing the degree of dehydration can be done using easily observed signs and symptoms, obtaining a serum bicarbonate level may improve the accuracy of predicting serious dehydration. A value below 17 meq/L usually reflects more severe dehydration.18,19
Differential Diagnosis
The most common cause of acute childhood diarrhea is viral gastroenteritis. Chronic nonspecific diarrhea, also known as "toddler's diarrhea" is also a common cause of diarrhea in children between 1 and 2 years of age. Frequently, stools are relatively well formed in the morning but become looser as the day progresses. The stool often appears to contain undigested vegetable matter but is without blood, mucous, or excessive fat. Children continue to gain weight appropriately, provided that they are offered a normal diet. The pathophysiology of toddler's diarrhea may involve abnormal intestinal motility with decreased transit time and excessive fruit juice intake. Other common causes of childhood diarrhea are antibiotic-associated diarrhea, systemic infections, food poisoning, bacterial or parasitic enteritis, and fecal impaction with diarrhea. Less commonly, infants might have diarrhea due to adrenogenital syndrome, primary disaccharidase deficiency or opiate withdrawal, and older children might present with diarrhea from toxic ingestions. Particularly serious causes of diarrhea that need to be considered in the differential diagnosis are intussusception, hemolytic-uremic syndrome (HUS), pseudomembranous colitis, appendicitis, and toxic megacolon.
The presence of blood or mucous in the stool almost never occurs with viral gastroenteritis and should suggest bacterial or parasitic infections. Bacterial or parasitic enteritis also may be associated with foreign travel, exposure to poultry or other farm animals, or consumption of processed meat. Viral pathogens, including rotavirus, tend to cause serious gastroenteritis in children younger than 2 years of age, while bacterial and parasitic agents generally cause gastroenteritis in older children 2-4 years of age. Patients with bloody diarrhea who have taken antibiotics may have pseudomembranous colitis.
The differential diagnosis of vomiting and diarrhea includes other nongastrointestinal tract illnesses such as meningitis, bacterial sepsis, pneumonia, acute otitis media, urinary tract infections, trauma, congestive heart failure, toxic ingestions, diabetic ketoacidosis, and other metabolic disorders.
Vomiting in newborns should be taken seriously. Many young infants suffer from gastroesophageal reflux or excessive feeding volume, but pyloric stenosis, intestinal obstruction such as malrotation with volvulus, intussusception, esophageal or intestinal atresia, meconium ileus, inborn errors of metabolism, and increased intracranial pressure should be in the differential diagnosis also. Older infants and children might present with vomiting due to metabolic disorders, intracranial masses, peptic ulcer disease, cyclic vomiting, or Munchausen syndrome by proxy.
Management
Anitviral Therapy. No specific antiviral therapy is available.
Antibiotic Therapy. Antibiotics are not indicated.
Antidiarrheal Medications. Antidiarrheal agents should be avoided. They can cause significant side effects and they do not address the underlying causes of diarrhea. Opiates such as opiate-atropine combinations can be responsible for causing drowsiness, lethargy, nausea, opiate-induced ileus, toxic megacolon, and central nervous system depression, coma, and even death.20,21
Salicylate toxicity may be result from frequent dosing of bismuth subsalicylate.22
Antiemetics. Older antiemetic agents such as phenothiazines should be avoided. They might interfere with oral rehydration by causing sedation and there is potential for development of extrapyramidal reactions, including dystonic reactions and oculogyric crisis.23 Promethazine is associated with increased risk of respiratory depression and it should not be used in children younger than 2 years and should be used with caution in older children.
Two recent studies showed that newer antiemetic agent, ondansetron, a 5-HT3 serotonin receptor antagonist, either by the oral24 or intravenous25 route, can be effective in decreasing vomiting and limiting hospital admission in children with emesis associated with acute gastroenteritis. (This is an off label use of this drug.)
Probiotics. Studies show that use of probiotics, especially lactobacillus, might be helpful in preventing or reducing the severity and duration of diarrhea, as well duration of hospitalization, in children with gastroenteritis, especially that caused by rotavirus.26-31 Invasive disease, mainly bacteremia, sepsis, and endocarditis, caused by lactobacillus in patients receiving probiotics has been documented by case reports, primarily in immunocompromised or severely debilitated patients.32,33 This is very rare, however, and overall appropriate use of lactobacillus has proven to be safe and effective in normal hosts.
Oral Rehydration Therapy. The mainstay of treatment of acute rotavirus gastroenteritis is Oral Rehydration Therapy (ORT) with glucose-electrolyte solutions, and early introduction of breast or formula feeding, and regular foods. Standard Oral Rehydration Solutions (ORS) contain sodium and potassium salts and glucose. A variety of over-the-counter ORS are available: Pedialyte, Enfalyte, Naturalyte, Rehydralyte, and WHO/UNICEF oral rehydration salts.
Both the American Academy of Pediatrics (AAP) and the Centers for Disease Control and Prevention (CDC) recommend oral rehydration as the preferred treatment of fluid and electrolyte losses caused by diarrhea in children with mild to moderate dehydration. There has been resistance to the use of ORT in the United States despite its proven efficacy.34 ORT offers several advantages over intravenous therapy—lower cost, elimination of the need for IV line placement, and treatment can be done or continued at home. A recent clinical trial in a pediatric emergency medicine department demonstrated that ORT is as effective as and superior to IVF for rehydration of moderately dehydrated children due to gastroenteritis. The therapy was initiated more quickly for ORT patients and patients treated with ORT had fewer hospitalizations.34 A recent meta-analysis of oral rehydration vs. IV therapy noted significantly fewer adverse events, less cost, and shorter length of hospital stay in those who received ORT.35 A nasogastric tube can be used in children who refuse to drink ORS. Continuous, slow administration of ORS via nasogastric tube might prevent hospitalization and has proven to be well tolerated, more cost-effective, and associated with fewer complications in comparison to IV hydration.36 Almost all children who have vomiting and dehydration can be treated with ORT. The key to therapy is to administer small volumes to avoid accumulation of a large amount of fluid in the stomach that might trigger vomiting. It can begin with 5 mL (1 teaspoon) aliquots every 1-2 minutes. This method is labor intensive, but it can successfully deliver 150-300 mL of fluids per hour. IV therapy should be reserved for the most severe cases.
Contraindications to oral rehydration therapy include:
- altered mental status with concern for aspiration;
- abdominal ileus; and
- severe dehydration.
Diet. When oral hydration therapy is complete, regular feeding with an age-appropriate diet should be resumed. Early re-feeding is recommended in managing acute gastroenteritis because oral feedings facilitate mucosal repair following rotavirus infection. Severe malnutrition can occur after gastroenteritis if prolonged gut rest or clear fluids are prescribed. Breastfed babies should continue to breastfeed while receiving ORT. If the infant is formula fed he/she should receive ORT for 12-24 hours and then return to formula feeding. Older children can begin eating within 12-24 hours after starting ORT. Foods like complex carbohydrates (rice, wheat, potatoes, bread, cereals) and lean meats, yogurt, fruits, and vegetables, are better tolerated that others. Foods high in simple sugars (including tea, juices, and soft drinks) should be avoided because of the risk of worsening osmotic diarrhea. At least 80% of children tolerate full strength animal milk or animal milk formula safely. The so-called BRAT diet (bananas, rice, applesauce, and toast) is discouraged because it is low in energy, density, protein, and fat.
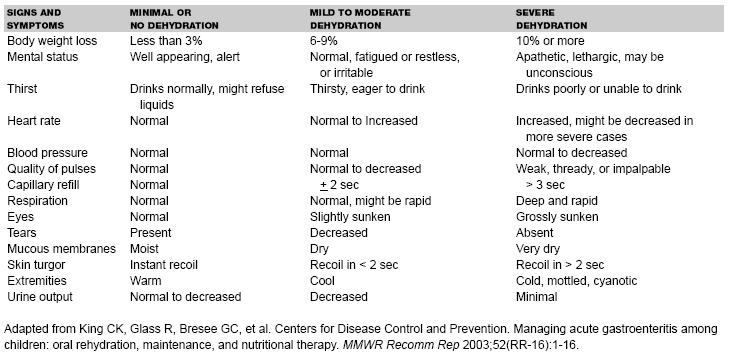
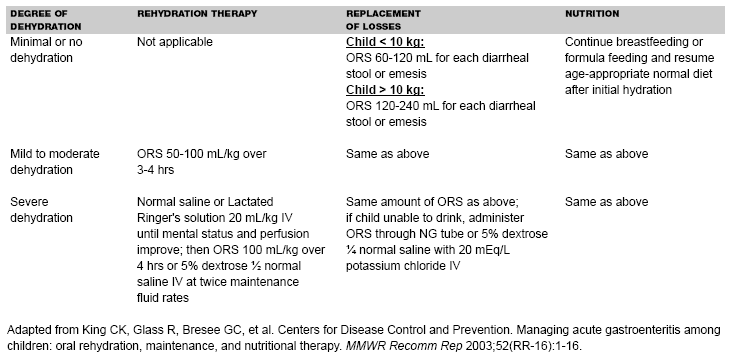
Management Guidelines. In 1996, the American Academy of Pediatrics and the CDC37 formulated clinical practice guidelines regarding the management of acute gastroenteritis in young children. Patients were divided into subgroups of mild (3-5% fluid deficit), moderate (6-9% fluid deficit), and severe (10% or more fluid deficit) dehydration. Studies showed that the first signs of dehydration might not be evident until 3-4% dehydration, with more clinical signs evident at 5% dehydration, and distinguishing between mild and moderate dehydration on the basis of clinical signs alone might be difficult.38,39 Updated recommendations prepared by CDC in 2003 group together patients with mild to moderate dehydration (see Table 1) and recommend oral solutions of lower osmolarity (75 mEq/L sodium, 75 mEq/L glucose, and total osmolarity of 245 mOsm/L), which have been associated with less vomiting, less diarrhea, and reduced need for IV hydration in comparison to standard ORS.40 Treatment should include two phases: a rehydration phase, in which the fluid deficit is replaced quickly over 3-4 hours, and a maintenance phase, which includes replacement of ongoing fluid and electrolyte losses and adequate dietary intake. (See Table 2.) Patients should be observed until signs of dehydration subside, because certain children with mild to moderate dehydration will not improve with ORT. After dehydration is corrected, further management can be implemented at home, provided that the child's caregivers understand home rehydration techniques, indications for returning for further evaluation, and have the means to do so.
| Table 1. Clinical Signs and Symptoms of Varying Degrees of Dehydration in Children19,20,39,40,41,42 |
|
| Table 2. Summary of Treatment Based on Degree of Dehydration |
|
Dehydration is most objectively measured as a change in weight from baseline, for example a 2-kg weight loss reflects the loss of 2 L of fluid. However, a previous recent weight is not available in many cases. When recent premorbid weight is unknown but a previous growth curve is available, an estimate of fluid loss can be obtained by subtracting current weight from expected weight as determined on the basis of the previous weight-for-age percentile. Clinical signs and symptoms are used to assess the severity of dehydration in most patients. (See Table 1.)
The most precise method of assessing fluid deficit is based on preillness weight, calculated as follows:
Fluid deficit ( L ) = preillness weight ( kg ) - illness weight (kg)
Percent dehydration = [( preillness weight - illness weight ) / preillness weight ] x 100%.
Minimal Dehydration. Provide adequate fluid and continue an age-appropriate diet. Encourage ORS. Use a teaspoon, syringe, or medicine dropper and give small volumes (e.g., 5 mL or 1 teaspoon) every 5 minutes and increase the volume gradually, as tolerated. Each diarrheal stool should be replaced with 10 mL/kg of ORS and each emesis should be replaced with 2 mL/kg body weight, or children weighing less than 10 kg should receive 2-4 oz. of ORS for each episode of diarrhea or emesis, and children above 10 kg should receive 4-8 oz.
Mild to Moderate Dehydration. Replace estimated fluid deficit rapidly 50-100 mL of ORS/kg during 3-4 hours and replace ongoing losses. Re-evaluate hydration status and replace fluid losses at least every 2 hours. As soon as dehydration is corrected, feeding should begin with age-appropriate diet.
Severe Dehydration. Severe dehydration is a medical emergency and requires immediate IV hydration with 20 mL/kg body weight of isotonic solution such as normal saline or Lactated Ringer's (LR) solution until pulse, perfusion, and mental status return to normal. The rapid administration of hypotonic or hypertonic solutions for purpose of volume resuscitation can results in serious complications including death and is never recommended in pediatric patients. If necessary, place two IV lines or look for alternative access sites (e.g., intraosseous infusion). Frail or malnourished infants should receive smaller amounts of fluids (10 mL/kg body weight) because they have reduced ability to increase cardiac output and distinguishing dehydration from sepsis might be difficult.
Obtain electrolyte levels. Assess patient's condition frequently. If the child does not respond to rapid bolus rehydration, consider the possibility of an underlying disorder such as septic shock, toxic shock syndrome, myocarditis, pericarditis, or myocardiopathy.
When the patient's condition has stabilized and mental status is satisfactory, begin oral rehydration therapy, with the IV line in place until it is certain that IV therapy is no longer needed. When rehydration is complete, resume age-appropriate feeding.
Complications and Disposition
Dehydration and electrolyte disturbances are the major complications of rotavirus gastroenteritis and occur most often in infants because they have a higher body surface-to-volume ratio, a higher metabolic rate, relatively smaller fluid reserves, and they depend on others for fluid intake. Severe dehydration may lead to significant reduction in tissue perfusion, decreased tissue oxygen delivery, and development of lactic acidosis. Although the effects of inadequate perfusion are reversible initially, prolonged tissue hypoxia leads to disruption of critical biochemical processes and cellular function, resulting in cell death, end-organ damage, and failure of multiple organ systems.
Children with significant rotavirus diarrhea present usually with isonatremic dehydration, and their serum sodium is between 130 and 150 meq/L. However, up to 25% of patients with rotavirus gastroenteritis have hypernatremic dehydration and their serum sodium is greater than 150 meq/L, which reflects water loss in excess of solute loss.42,43 This usually occurs in children with diarrhea who also are febrile and have inadequate fluid intake. Those patients may be very agitated and irritable and the symptoms can progress to lethargy and coma. Physical exam frequently reveals thick and doughy skin with normal skin turgor, increased muscle tone, nuchal rigidity, and brisk reflexes. Children with serum sodium above 155 meq/L who are corrected too rapidly are at the greatest risk for cerebral edema resulting in seizures and permanent neurologic damage, or death. The goals of therapy are correction of fluid deficit and gradual correction of hypernatremia at the rate of less than 0.5 meq/L per hour.
Emergency Department Management:
Formula:
Free water deficit (in L) = (0.6)( weight in kg ) x [( actual serum Na+- desired serum Na+) / (actual serum Na+)]
Sample calculation: Child with 10% dehydration. Weight is 7.0 kg.
Measured serum sodium = 165 mEq/L
Desired serum sodium = 145 mEq/L
Calculation: (0.6)(7.0) x [( 165 - 145 ) / 165] = 0.58 L = 580 mL
Child is 10% dehydrated, (10%)(7.0 kg)= 700 mL is the deficit. Of that amount, 580 mL is free water and remainder—120 mL should be given as normal saline.
Another example:
Child with 10% dehydration. Weight is 10.0 kg.
Measured serum sodium = 168 mEq/L
Desired serum sodium = 145 mEq/L
Calculation: (0.6)( 10.0) x [( 168 - 145 ) / 168] = 0.82 = 820 mL
Child is 10% dehydrated, (10%)(10.0 kg)= 1000 mL is the deficit. Of that amount, 820 mL is free water and remainder—180 mL should be given as normal saline.
Begin with fluid resuscitation using 0.9% normal saline before correcting the free water deficit, and monitor serum sodium. If serum sodium does not drop by 0.5 mEq/L per hour, change your IV fluids to three-quarters normal saline, and if the rate of serum sodium decrease still does not drop by 0.5 mEq/L per hour, change IV fluids to half saline, until you reach your goal of slow and steady decrease of serum sodium. Some authors recommend using 0.45% NaCl in 5% D5W (half normal saline in 5% dextrose in water) for water deficit replacement for children with gastroenteritis and hypernatremia due to sodium and water loss.44 Allow oral hydration as soon as it can be tolerated safely. Patients who have acute hypernatremia that is corrected by the oral route can tolerate a more rapid rate of correction with a much lower incidence of seizures.44 The water deficit should be replaced over more than 36 hours and the serum sodium concentration must be monitored.
Hyponatremia in dehydrated patients can be caused by intake of hypotonic solutions. Serum sodium in patients with hyponatremic dehydration is less than 130 meq/L. A major consequence of hyponatremia is influx of water into the intracellular space, resulting in cellular swelling that can lead to cerebral edema and encephalopathy. When the sodium level falls acutely below 125 meq/L patients become symptomatic (nausea, malaise, headache) and when the level falls below 120 meq/L, lethargy, obtundation, and seizures occur. Advanced symptoms include signs of cerebellar herniation, such as respiratory arrest, dilated pupils, and decorticate posturing, and they can develop suddenly. Children are at particularly high risk for developing symptomatic hyponatremia.They develop hyponatremic encephalopathy at higher sodium concentrations that do adults and have a poor prognosis if the therapy is not initiated quickly. This appears to be due to the higher brain-to-skull size ratio in children, which leaves less room for brain expansion.44
Most patients with mild to moderate hyponatremia can be treated with isotonic saline alone, which will correct the volume depletion and raise the serum sodium. The goals of therapy are correction of fluid deficit and gradual correction of hyponatremia at the rate of 0.5 mEq/L sodium per hour. In general, if there are no neurologic manifestations of hyponatremia, correction with hypertonic saline is unnecessary and potentially harmful. Symptomatic hyponatremia, on the other hand, is a medical emergency.
Emergency Department Management of Symptomatic Patients. Administer hypertonic saline (3% normal saline) by an infusion pump. The rate of infusion should raise the plasma sodium concentration by about 1 mEq/L per hour until either the patient becomes alert and seizure-free, the plasma sodium level increases by 20-25 mEq/L, or serum sodium concentration level reaches 125-130 mEq/L, whichever occurs first. If the patient is seizing or showing other signs of increased intracranial pressure, increase the rate of infusion to raise the serum sodium level by 4-8 mEq/L during the first hour or until the seizure stops. One mL/kg of 3% saline will raise the plasma sodium by about 1 mEq/L.44
Overly rapid correction of hyponatremia can lead to the development of osmotic demyelination and irreversible brain damage. Cerebral demyelination lesions are rare, however. They typically occur several days following correction of hyponatremia and can present with confusion, quadriplegia, pseudobulbar palsy, and a pseudocoma with a "locked in" stare.44 Because the risk of death and permanent neurologic damage is greater than the possibility of demyelinating lesions following corrections, we should not hesitate to use hypertonic saline in symptomatic patients.
Formula:
Sodium deficit [ mEq] = ( weight in kg)(0.6)( desired serum Na+ - actual serum Na+ )
Sample calculation: A seizing child with serum Na+ = 110 mEq/L
Weight = 10 kg
Desired serum Na+ = 120 mEq/L (seizure typically stops at the serum Na+ level of 120 mEq/L)
Sodium deficit = (10 kg) (0.6) (120 mEq/L - 110 mEq/L) = 60 mEq
Sodium contents:
1 liter of 0.9% saline = 154 mEq of sodium
1 liter of 3% saline = 513 mEq of sodium
Therefore, 60 mEq of sodium would require 120 mL of 3% saline.
Start by using one-third of the amount (one-third of 120 mL is 40 mL) = 40 mL of 3% saline IV, over 5-10 min, repeat twice, if needed, until the seizure stops.
The goal is correction of hyponatremia at 0.5 mEq/L sodium per hour, once the patient is stable.
Four cases of bacteremia as a complication of rotavirus disease were recently described in one neonate and three infants.45 All had an increase in temperature several days after rotavirus gastroenteritis. The causative organisms were Enterobacter cloacae and Klebsiella pneumoniae. All four patients had full recovery. Damage to the vulnerable intestinal mucosa occurring during rotavirus disease can enable enteric bacteria to invade the bloodstream, especially in younger infants. Although this complication is rare, when a second peak of fever with no obvious source occurs in infants with rotavirus gastroenteritis, blood cultures should be obtained and empiric antibiotic therapy should be considered pending the culture results.
Chronic infections can occur in immunodeficient children, and disease manifestations can be more severe in malnourished hosts. Rotaviruses have been associated with kidney and liver infections and damage in immunocompromised children.46
Admission to the hospital is necessary if caregivers cannot provide adequate care at home, if the child demonstrates unusual irritability or drowsiness, or dehydration worsens despite adequate ORT, or there is a concern about other illness complicating the clinical course. Children with severe dehydration, especially young ones, should be admitted to the hospital for close monitoring. Hospitalization also is indicated if there is a significant difficulty with administering oral rehydration therapy, such as intractable vomiting, ORS refusal, or inadequate ORS intake.
Prevention and Vaccine Development
Unlike many respiratory viruses, rotavirus is relatively resistant to disinfection, and transmission may occur despite routine hand washing. Studies have shown that hand washing with soap and water removes only 75% of virus load from hands. Skin disinfectants such as chlorhexidine and general disinfectants such as bleach are ineffective against rotavirus. It appears that the most effective anti-septic agents are alcohol-based. To control the spread of rotavirus, visible stool should be cleansed from the hands with soap and water and then an alcohol-containing hand rub should be used. Potentially contaminated surfaces, such as changing tables, should be cleaned of all visible stool and then disinfected with 95% ethanol or another alcohol-containing disinfectant.47 Education of caregivers about appropriate hygienic measures, cohorting of children, and isolation of ill children are successful preventative measures in all types of childcare situations and hospitals.
The most effective preventive measure is vaccination. Research to develop a safe and effective rotavirus vaccine began in mid-1970s. The first rotavirus vaccine in the United States, RotaShield, was licensed by the FDA on August 31, 1998, and shortly thereafter was given routinely to infants at ages 2, 4, and 6 months. Although the vaccine was highly effective in preventing severe rotavirus gastroenteritis and dehydration, several cases of vaccine-associated intussusception were reported. The period of greatest risk for intussusception was 3-10 days after the first of three doses. After epidemiologic studies confirmed an association between intussusception and RotaShield, the manufacturer voluntarily removed RotaShield from the U.S. market nine months after it was introduced.
RotaTeq is a pentavalent ( human-bovine ) reassortant rotavirus vaccine. It is based on a bovine strain, WC3, which is naturally attenuated for humans, but not broadly cross-protective. Each reassortant virus contains a single gene encoding a major outer capsid protein from the most common human serotypes: G1, G2, G3, G4, and P(8). A large-scale study assessed safety and efficacy of RotaTeq.48 The efficacy of the vaccine against severe G1-G4 rotavirus gastroenteritis was 98%, and against G1-G4 rotavirus gastroenteritis of any severity was 74%. The vaccine reduced hospitalization rate for G1-G4 gastroenteritis by 96%. The efficacy of the vaccine against all gastroenteritis-related hospitalizations after the first dose was 59%. The emergency department visits related to G1-G4 rotavirus gastroenteritis were reduced by 94% and clinic visits by 86%. There was no significant difference between patients given vaccine or placebo in development of intussusception during the one-year follow-up. RotaTeq was approved by the FDA on February 3, 2006. On February 21, 2006, the Advisory Committee on Immunization Practices (ACIP) to the Centers for the CDC recommended for the inclusion of RotaTeq in the U.S. vaccine schedule. It requires three doses and it is administered orally at 2, 4, and 6 months of age.
Rotarix is a live-attenuated human rotavirus vaccine. It is a monovalent vaccine derived from the most common human rotavirus strain (RIX4414) of G1P(8) specificity that has been attenuated by serial passage. It provides at least some cross-protection against most other serotypes. Clinical trail conducted in Latin American countries and in Finland showed that Rotarix was 85% effective in preventing severe rotavirus gastroenteritis and 40% effective against severe gastroenteritis from any cause.49 The efficacy against gastroenteritis on account of G1P(8) strain was 92% and against strains sharing only the P(8) antigen was 87%. The efficacy against hospitalization for rotavirus gastroenteritis was 85% and against hospitalization for gastroenteritis from any cause was 42%. There was no increased risk of intussusception in the vaccine group compared with the placebo group. Rotarix is currently in phase three of development in the United States. It is licensed in several countries. It requires two doses and it is administered orally at 2 and 4 months of age.
Summary
Rotavirus remains the most common cause of severe gastroenteritis in children in the United States and around the world. Recent developments in the science of gastroenteritis management provide evidence that the combination of oral rehydration therapy and early re-feeding and nutritional support with age-appropriate diet is effective in treating acute diarrhea due to rotavirus disease. Oral rehydration therapy is the preferred treatment for children with mild and moderate dehydration due to rotavirus gastroenteritis. Advantages of oral rehydration include lower cost, elimination of the need for IV placement, and involvement of the parents in treatment, which they can continue at home. Vaccination, however, is the only practical way to gain control over rotavirus gastroenteritis. In February 2006, a new rotavirus vaccine, RotaTeq, was implemented in the childhood immunization schedule in the United States. Another new rotavirus vaccine, Rotarix, is currently in phase three of development in the United States. Both vaccines seem safe and effective in inducing protective immunity against severe rotavirus disease and we should expect to see a significant reduction in hospitalizations, outpatient visits, and parents' workdays lost due to winter gastroenteritis.
References
1. Ho MS, Glass RI, Pinsky PF, et al. Rotavirus as a cause of diarrheal morbidity and mortality in the United States. J Infect Dis 1988;158:1112-1116.
2. Parashar UD, Hummelman EG, Bresse JS, et al. Global illness and deaths caused by rotavirus disease in children. Emerg Infect Dis 2003;9:565-572.
3. Anderson EJ, Weber SG. Rotavirus infection in adults. Lancet Infect Dis 2004;4:91-92.
4. Newman RD, Grupp-Phelan J, Shay DK, et al. Perinatal risk factors for infant hospitalization with viral gastroenteritis. Pediatrics 1999;103:E3.
5. Velazquez FR, Matson DO, Calva JJ, et al. Rotavirus infections in infants as protection against subsequent infections. N Engl J Med 1996;335:1022.
6. Ward RL, Bernstein DI, Young EC, et al. Human rotavirus studies in volunteers: Determination of infectious dose and serological response to infection. J Infect Dis 1986;154:871-880.
7. Keswick BH, Pickering LK, DuPont HL, et al. Survival and detection of rotaviruses on environmental surfaces in day care centers. Appl Environ Microbiol 1983;46:813-816.
8. Barlett AV, Reves RR, Pickering LK. Rotavirus in infant-toddler day care centers: Epidemiology relevant to disease control strategies. J Pediatr 1988;113:435-441.
9. Ramig RF. Pathogenesis of intestinal and systemic rotavirus infection. J Virol 2004;78:10213.
10. Lundgren O, Peregrin AT, Persson K, et al. Role of the enteric nervous system in the fluid and electrolyte secretion of rotavirus diarrhea. Science 2000;287:491-495.
11. Ball JM, Tian P, Zeng CQ, et al. Age-dependent diarrhea induced by a rotaviral nonstructural glycoprotein. Science 1996;272:101-104.
12. Staat MA, Azimi PH, Berke T, et al. Clinical presentations of rotavirus infection among hospitalized children. Pediatr Infect Dis J 2002;21:221.
13. Khoshoo V, Bhan MK, Jayashree S, et al. Rotavirus infection and persistent diarrhea in young children. Lancet 1990;336:1314-1315.
14. Littlewood JM. 66 infants with urinary tract infection in first month of life. Arch Dis Child 1972;47:218.
15. Drew JH, Acton CM. Radiological findings in newborn infants with urinary infection. Arch Dis Child 1976;51:628-630.
16. Maherzi M, Guignard JP, Torrado A. Urinary tract infection in high-risk newborn infants. Pediatrics 1978;62:521.
17. Bergstrom T, Larson H, Lincoln K, et al. Studies of urinary tract infections in infancy and childhood. XII. Eighty consecutive patients with neonatal infection. J Pediatr 1972;80:858.
18. Steiner MJ, DeWalt DA, Byerley JS. Is this child dehydrated? JAMA 2004;291:2746.
19. Vega RM, Avner JR. A prospective study of the usefulness of clinical and laboratory parameters for predicting percentage of dehydration in children. Pediatr Emerg Care 1997;13:179.
20. Brown JW. Toxic megacolon associated with loperamide therapy. JAMA 1979;241:501.
21. Curtis JA, Goel KM. Lomotil poisoning in children. Arch Dis Child 1979;54:222.
22. Pickering LK, Feldman S, Ericsson CD, et al. Absorption of salicylate and bismuth from a bismuth subsalicylate containing compound (Pepto-Bismol). J Pediatr 1981;99:654.
23. Carey MJ, Aitken ME. Adverse effects of antiemetics in children. N Z Med J 1994;107:452.
24. Ramsook C, Sahagun-Carreon I, Kozinetz CA, et al. A randomized clinical trial comparing oral ondansetron with placebo in children with vomiting from acute gastroenteritis. Ann Emerg Med 2002;39:397-403.
25. Reeves JJ, Shannon MW, Flesher GR. Ondansetron decreases vomiting associated with acute gastroenteritis: A randomized, controlled trial. Pediatrics 2002;109:e62.
26. Vanderhoof JA, Young RJ. Use of probiotics in childhood gastrointestinal disorders. I Pediatr Gastroenterol Nutr 1998;27:323-32.
27. Guandalini S, Pensabene L, Zikri MA, et al. Lactobacillus GG administered in oral rehydration solution to children with acute diarrhea: A multicenter European trial. I Pediatr Gastroenterol Nutr 2000;30:54-60.
28. Rosenfeld V, Michaelsen KF, Jakobsen M, et al. Effect of probiotic Lactobacillus strains in young children hospitalized with acute diarrhea. Pediatr Infect Dis J 2002;21:411-416.
29. Van Niel CC, Feudner C, Garrison MM, et al. Lactobacillus therapy for acute infectious diarrhea in children: A meta analysis. Pediatrics 2002;109:678-684.
30. Guandalini S, Pensabene L, Zikri MA, et al. Lactobacillus GG administered in oral rehydration solution to children with acute diarrhea: A multicenter European trial. J Pediatr Gastroenterol Nutr 2000;30:54-60.
31. Rosenfeld V, Michaelsen KF, Jakobsen M, et al. Effect of probiotic Lactobacillus strains on acute diarrhea in a cohort on nonhopitalized children attending day-care centers. Pediatr Infect Dis J 2002;21:417-419.
32. Land MH, Rouster-Stevens K, Woods CR, et al. Lactobacillus sepsis associated with probiotic therapy. Pediatrics 2005;115:178-181.
33. De Groote MA, Frank DN, Dowell E, et al. Lactobacillus rhamnosus GG bacteremia associated with probiotic use in a child with short gut syndrome. Pediatr Infect Di J 2005;24:278-280.
34. Spandorfer PR, Alessandrini EA, Joffe MD, et al. Oral versus intravenous rehydration of moderately dehydrated children: A randomized, controlled trial. Pediatrics 2005;115:295-301.
35. Fonseca BK, Holdgate A, Craig JC. Enteral vs intravenous rehydration therapy for children with gastroenteritis. A meta-analysis of randomized controlled trials. Arch Pediatr Adolsec Med 2004;158:483-490.
36. Nager AL, Wang VJ. Comparison of nasogastric and intravenous methods of rehydration in pediatric patients with acute dehydration. Pediatrics 2002;109:566-572.
37. American Academy of Pediatrics. Practice parameter: The management of acute gastroenteritis in young children. Pediatrics 1996;97:424-435.
38. World Health Organization. The treatment of diarrhea: A manual for physicians and other senior health workers. Geneva, Switzerland: World Health Organization, 1995.
39. Duggan C, Refat M, Hashem M, et al. How valid are clinical signs of dehydration in infants? J Pediatr Gastroenterol Nutr 1996;22:56-61.
40. King CK, Glass R, Bresee GC, et al. Centers for Disease Control and Prevention. Managing acute gastroenteritis among children: Oral rehydration, maintenance, and nutritional therapy. MMWR Recomm Rep 2003;52(RR-16):1-16
41. Sandhu BK. Practical guidelines for the management of gastroenteritis in children. J Pediatr Gastroenterol Nutr 2001;33(Suppl 2):S36-39.
42. Rodriguez WJ, Kim HW, Arrobio JO, et al. Clinical features of acute gastroenteritis associated with human reovirus-like agent in infants and young children. J Pediatr 1977;91:188.
43. Kovacs A, Chan L, Hotrakitya C, et al. Clinical and laboratory features and use of the Rotazyme test. Am J Dis Child 1987;141:161.
44. Moritz ML, Ayus JC. Disorders in water metabolism in children: Hyponatremia and hypernatremia. Pediatr Rev 2002;23:371-380.
45. Lowenthal A, Livni G, Amir J, et al. Secondary bacteremia after rotavirus gastroenteritis in infancy. Pediatrics 2006;117:178-181.
46. Gilger MA, Matson DO, Conner EM, et al. Extraintestinal rotavirus infections in children with immunodeficiency. J Pediatr 1992;120:912.
47. Zerr DM, Allpress AL, Heath J, et al. Decreasing hospital-associated rotavirus infections: A multidisciplinary hand hygiene campaign in a children's hospital. Pediatr Infec Dis J 2005;24:397.
48. Vesikari T, Matson DO, Dennehy P, et al. Safety and efficacy of pentavalent human-bovine (WC3) reassortant rotavirus vaccine in preventing rotavirus gastroenteritis and reducing associated healthcare resource utilization. N Engl J Med 2006;354:23-33.
49. Ruiz-Palacios GM, Perez-Schael I, Raul Velazquez F, et al. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med 2006;354:11-21.
In North America, rotavirus gastroenteritis is a predictable winter epidemic every year--young children presenting to emergency departments and outpatient clinics with fever, vomiting, diarrhea, and varying degrees of dehydration.Subscribe Now for Access
You have reached your article limit for the month. We hope you found our articles both enjoyable and insightful. For information on new subscriptions, product trials, alternative billing arrangements or group and site discounts please call 800-688-2421. We look forward to having you as a long-term member of the Relias Media community.