Influenza: Old Illness, New Fear
Influenza: Old Illness, New Fear
Authors: Kenneth H. Butler, DO, FACEP, FAAEM, Associate Professor, Associate Residency Director, University of Maryland Emergency Medicine Residency Program, University of Maryland School of Medicine, Baltimore; and Yael R. Goldfeder, MD, Division of Emergency Medicine, Department of Surgery, University of Maryland School of Medicine, Baltimore.
Peer Reviewers: Sandra Schneider, MD, FACEP, Professor and Chair, Department of Emergency Medicine, University of Rochester, NY; and Steven Winograd, MD, FACEP, Attending Physician, Emergency Department, Adena Regional Medical Center, Chillicothe, OH.
History
Multiple influenza pandemics throughout the years have prompted intense research into the diagnosis, treatment, and prevention of influenza virus infection. The first documented pandemic occurred in 1580 in Asia and caused widespread disease. There were several remarkable pandemics during the 20th century. The most overwhelming was the Spanish flu of 1918, subtype A(H2N2), which caused 20-40 million deaths, mostly among people ages 20-40 years. In the United States, the death toll was more than 500,000, and the disease killed people within days of infection. The bulk of the devastation occurred in less than 1 year. In 1932, the first influenza virus (types A, B, and C) was isolated and identified in the laboratory. In 1957, influenza A(H2N2) emerged in southern China and spread to the United States, causing 70,000 deaths, mostly among the very young and the very old. In 1968, Hong Kong experienced a mild pandemic, which spread to the United States; this strain tended to affect young adolescents but caused the greatest mortality among the elderly. Thirty-thousand Americans perished. In the late 1990s, concern arose in Asia because of a new strain of influenza, H5N1, that primarily affected the avian population but was confirmed in humans as well. Avian-to-human transfer was new and is still a topic of alarm.1,2
Epidemiology
Influenza infection peaks in the winter months. During the winter of 2004-2005, the prevalence of infection peaked in early February with a moderate amount of overall activity. Seventy-nine percent of the isolates were influenza A, and of those, 99.7% were A(H3N2). Twenty-one percent of the viruses were influenza B, and that percentage increased as the season progressed.3
Influenza is associated with more than 34,000 deaths per year in the United States.4 A disproportionate number of these deaths occur in patients older than 65 years because they have more comorbidities than do younger patients. They also constitute an ever-increasing percentage of the total population.4 During the 2004-2005 influenza season, 2.6% of all health care visits in the United States were for influenza-like illnesses. Twenty-four children died of laboratory-confirmed influenza, and 8.6% of all deaths during the influenza season were attributed to influenza and pneumonia.3 During an epidemic, 5-30% of the population may be infected. Yearly costs associated with the influenza virus reach $11-18 billion in the United States. Contributing to this amount are health care costs and days of work lost.5
Between 1979 and 2001, the number of influenza-associated illnesses increased each year among Americans older than 50 years. Increases in the number of hospitalizations for respiratory and cardiovascular episodes secondary to influenza also were noted for children younger than 5 years and adults older than 65 years. During this period, hospital admissions for influenza averaged almost 95,000 per year. The number of cardiopulmonary diagnoses listed with influenza diagnoses approximated 226,000 admissions per year. These increases have been attributed in part to the aging population, the prevalence of influenza A(H3N2), as well as the lengthening of the overall influenza season in the 1990s.6 During the 2003-2004 season, which was a type A(H3N2) season, 152 children died from influenza. As a result, in June 2004, childhood death secondary to influenza infection became a reportable illness.6
People of all age groups are susceptible to influenza and its complications, but many factors influence the morbidity and mortality associated with the disease. Prevalence is increased in populations with a high percentage of elderly, among people who travel, and in dense urban communities. Many people who lack medical insurance do not get immunized and therefore are at increased risk of infection. School-age children are the conduits by which many viruses, including influenza, spread. Models have indicated that targeting school children for immunization would be as effective in protecting the population as immunizing the priority groups currently under recommendation.1,7
Certain conditions are predictive of populations at increased risk for hospitalization for influenza-related reasons. These include malignancies, chronic heart disease, diabetes mellitus, and renal dysfunction. A high risk of hospitalization also is associated with the incurrence of high health care fees in the months preceding an influenza outbreak (suggesting comorbidities).8
Biology of the Influenza Virus
The influenza virus is a 110-nm enveloped single-stranded RNA virus in the Orthomyxoviridae family. The notable types are influenza A, which infects multiple species; influenza B, which infects only humans; and influenza C, which causes mild human illness. These differences are based on core protein differences.9 Influenza A has 8 single-stranded RNA segments. The two major glycoproteins on the surfacehemagglutinin (HA or H) and neuraminidase (NA or N)confer the antigenic properties of the specific virus. Only influenza A has subtyping according to these surface proteins. There are 16 HA subtypes and 9 NA subtypes for influenza A. The human variants are typically H1, H2, or H3 and N1 or N2. Until recently, the others lived mostly in the avian population.2
The subtyping nomenclature for influenza A is structured as follows: host of origin/geographic location of the first isolation/strain number/year of isolation (antigenic description). For example, A/California/7/2004(H3N2) represents influenza A virus, strain number 7, first isolated in California in 2004, with H and N types.2
The influenza virus replicates primarily in the cells of the respiratory tract. Hemagglutinin's receptor binding site binds to sialic acid residues of various glycoproteins and lipids on host cell surfaces. The virus then undergoes receptor-mediated endocytosis into acidic endosomes. The low pH and the hemagglutinin cause the viral membrane to fuse with the cell membrane and release the viral RNA into the cytosol. The M2 protein forms an ion channel that is needed for viral uncoating. The RNA makes its way through nuclear pores into the host cell nucleus, where it uses host proteins to make its own viral mRNA and synthesizes new viral particles.5 Hours later, the new virus buds out of the host cell.10 The neuraminidase of the virus prevents it from remaining attached to the host cell upon budding from it by destroying the sialic acid-containing receptors on the host cell.2
The respiratory tract sustains the brunt of the virus and, therefore, is the primary site for defense. Once infected, epithelial cells along the respiratory tract necrose. Cellular apoptosis occurs because the virus has halted cell protein synthesis. The columnar ciliated cells desquamate, and inflammatory cells infiltrate the submucosa, causing edema and hyperemia. Type I and II alveolar epithelial cells are affected. They also exhibit edema, exudates, and hemorrhage, leading to alveolar rupture. The epithelial cells produce the major inflammatory cytokines, interferon-alpha and interleukin-6. Their concentrations peak on day 2 of infection, correlating with the severity of illness.5
The immunocompetent host has multiple mechanisms of protection from the virus. The nearly immediate innate immune system response consists of NK cells, macrophages, interferon, cytokines, fever, and mucus, which destroy most of the virus within hours. The primary humoral antibody response is protective and antigen specific. It includes IgA in addition to IgM and IgG. IgA induces a local mucosal response and is a key factor in protecting against infection as well as increasing clearance of the virus after infection. A cellular response, primarily involving CD4+ T helper cells, aids B cells in the production of antibodies. CD8 cytotoxic T cells kill host cells containing the virus, although this process is not completely understood.5,10
Clinical Features
During an infection, aerosolized droplets carrying viral particles are disseminated through sneezing and coughing. The particles are less than 2 micrometers in diameter and, when inhaled, can reach the lower respiratory tract. Viral shedding peaks at 48 hours after exposure and continues for 6-8 days.4 The greatest communicability occurs during the first 3 days of illness. Virus can shed even before symptom onset and up to 7 days after infection.11
Patients present with various nonspecific and systemic symptoms. No grouping of signs or symptoms can rule in or out a diagnosis of influenza. Typical signs and symptoms, however, are highly suggestive of influenza, including fever, headache, cough, and myalgia in a patient who presents within 2 or 3 days of symptom onset.12 Upper respiratory infection symptoms such as rhinorrhea and nasal obstruction are present initially, leading to photophobia and eye pain with lateral motion. Other symptoms include sore throat, nonproductive cough, rigors, and sweats. Many of these symptoms overlap with those of parainfluenza and respiratory syncytial virus (RSV). Abdominal symptoms are less common in adult patients. Fever will increase 12 hours after illness onset, and fever lasts an average of 3 days, although its duration can be 1-5 days. On presentation, the patient likely will appear toxic and will be warm and flushed. Lymphadenopathy may be apparent on examination, and fewer than 25% of patients have abnormal pulmonary findings.13
Risk stratification must be used in the clinical assessment of influenza. A cluster of symptoms vs. a single symptom should be considered when making a likelihood determination of the disease in a particular patient. If a patient describes a lack of systemic symptoms and cough, is able to conduct daily activities, and is not confined to bed, the likelihood of influenza drops dramatically.14 Clinicians can increase the likelihood of diagnosing influenza if they are aware of the prevalence of influenza in their region during that specific period. This information is available from the Centers for Disease Control and Prevention at www.cdc.gov/flu/weekly/.
Seventy-five percent of patients have a sudden onset of symptoms that quickly become systemic.13 In a study conducted by Zambon et al,15 physicians were able to diagnose 77% of cases of laboratory-confirmed influenza using the definition of fever of 37.8°C or greater and the presence of two signs/symptoms (such as headache, myalgias, sore throat, or cough) in patients presenting within 48 hours of onset during a period of high influenza prevalence. Call et al12 concluded that the stronger predictor of influenza was the sudden onset of fever and cough in patients older than 60 years. Another study of the ability of clinical diagnosis to accurately predict actual influenza cases revealed sensitivities of 80-86% but specificity of only 35-58%.16 At this time, no formal practice has been validated to diagnose influenza by symptoms.14
Diagnostic Testing
Accurate and early detection of influenza is important on an individual and a population level. Epidemiologic data depend on accurate results. These data are important for vaccination composition, tracking resistant strains, and utilization of scarce resources in anticipation of and during epidemics. Proper diagnosis can de-crease an individual's emergency department (ED) and hospital length of stay (LOS),17 decrease antibiotic usage, decrease ancillary testing, and aid in effecting appropriate isolation.17-19 A clinical definition for influenza would be helpful in determining which patients truly have the illness without the need for testing. Because so many other illnesses, such as RSV, parainfluenza, and adenovirus, present during the same season with similar symptoms, it can be difficult to accurately identify patients with influenza.
Various methods of testing for influenza have been compared to the gold standard of viral culture. This gold standard is not particularly useful for individual patients because, by the time results are back, their symptoms usually are resolving. Viral culture, however, is helpful in identifying the strains that are circulating and the resistance patterns. The results of testing methods with shorter turnaround times, e.g., fluorescent antibody staining and rapid diagnostic tests, usually are confirmed by viral culture.
Fluorescent antibody staining is performed with samples obtained from nasopharyngeal swabs or aspirates or nasal swabs. In the direct immunofluorescent antibody (DFA) test, a fluorescein-tagged antibody is used to detect and bind viral antigen. The complex then is detected by fluorescent microscopy. In the indirect immunofluorescent antibody (IFA) test, an unlabeled antibody is bound to influenza antigen; an antibody conjugated to a fluorescein compound is used to bind to the original antibody-antigen complex and then is detected with a fluorescent microscope. Both tests require an adequate specimen of epithelial cells and technician experience. They must be done in a hospital or reference laboratory, and results become available in 2-4 hours. Fluorescent antibody staining has a sensitivity of 40-90% and a specificity of 86-100% compared with viral culture.20 The current standard is an immunofluorescent test and viral culture confirmation, which can take 2-14 days.18
The newer rapid diagnostic tests can be completed in fewer than 30 minutes. These immunoassays detect viral antigens or detect neuraminidase activity. (See Table 1.)21 Each test can be run with various specimens, including nasopharyngeal swab, nasal wash or aspirate, and throat swab.21 The sensitivities and specificities vary from 40-100% and 52-100%, respectively.12 The positive predictive value (PPV) and the negative predictive value (NPV) are highly dependent on the season and the prevalence of influenza in the population. PPVs all increase during peak influenza activity.
Reverse transcriptase polymerase chain reaction (RT-PCR) has a sensitivity of 93% for influenza,22 and results can be obtained in 6 hours. Multiplex PCR is at least as sensitive as viral culture.18 The multiplex version of the PCR can confirm conditions being considered in the differential diagnoses as well. In fact, from throat swab specimens, it was not only able to detect dual infections (influenza, RSV, and parainfluenza) but also detected 23% more positive infections than viral culture. Although not as rapid as the antigen detection tests, it is more sensitive and specific and does not require viral culture for negative confirmation.18
Other testing for patients with possible influenza may include chest film, blood culture, sputum culture, and fingerstick, depending on comorbid conditions and symptoms on presentation.
Differential Diagnosis
Certain illnesses with a wide range of consequences can mimic influenza. These include RSV, parainfluenza, adenovirus, Mycoplasma pneumoniae, severe acute respiratory syndrome (SARS), and inhalational anthrax. The latter two are the most frightening on a global scale and always must be included in the differential. SARS is caused by a coronavirus first identified in China in 2002. The case definition is fever 38°C or higher, radiologic infiltrate consistent with pneumonia or ARDS without an identifiable cause, and no alternative diagnosis.23 Inhalational anthrax (IA) is the most lethal form of anthrax and initially presents like an influenza-like illness with fever, cough, myalgias, nausea, and vomiting. Other symptoms include dyspnea, pleuritic chest pain, and drenching sweats. The milder upper respiratory symptoms typical of influenza, i.e., sore throat and rhinorrhea, are not seen in IA. Patients with IA usually have a decreased pulse oximetry reading and an abnormal chest film with infiltrates, pleural effusions, and a widened mediastinum. Blood cultures reveal gram-positive rods within 24 hours. Early administration of antibiotics is key for treatment of patients who present with these symptoms and are considered high risk (i.e., after known or potential exposure).24,25
Management and Disposition
Treatment for influenza is largely supportive. If patients present early in their course, they may benefit from antiviral therapy. Most patients with influenza can be treated as outpatients with oral hydration, antipyretics, and anti-inflammatories. Antivirals should be considered in patients who will be discharged to home, particularly if they have close contact with high-risk patients or others who have not been vaccinated. The antivirals decrease the time to functional recovery by 1 to 1½ days. Patients requiring admission are those with viral pneumonia or secondary bacterial pneumonia requiring oxygen or ventilator support, those in need of intravenous hydration, and patients with cardiovascular complications secondary to influenza infection.26 Patients with exacerbations of high-risk conditions such as chronic obstructive pulmonary disease (COPD), asthma, diabetes, and renal dysfunction also warrant admission. Prior to dispensing antivirals to any patient, the clinician should consider a bacterial source of infection.
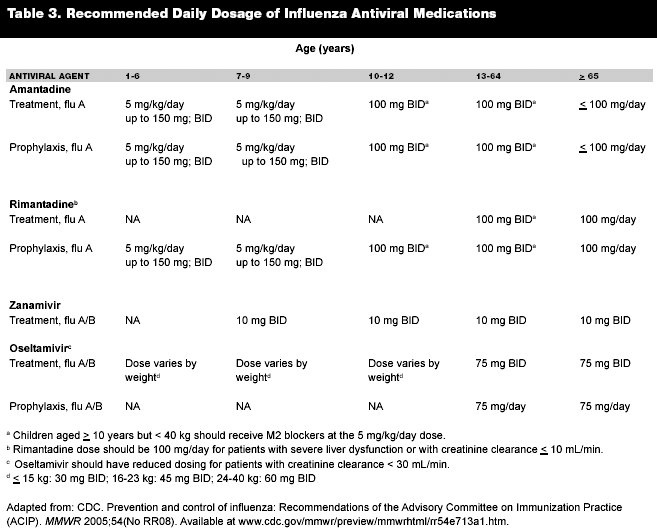
There are two major classes of antiviral agents. M2 protein blockers target the viral M2 protein, which aids in the uncoating of the virion within the host cell. Amantadine and rimantadine are the M2 blockers currently used. (See Table 2.) Both can be used for prophylaxis against and treatment of influenza A. They are most effective if started within 48 hours of symptoms onset; oral administration has good bioavailability. Side effects include nausea, dizziness, and insomnia. The gastrointestinal and central nervous system (CNS) side effects are more intense with amantadine (overall, rimantadine has a better side effect profile). Resistance to these medications, resulting from an amino acid substitution, is on the rise. Up to 30% of people treated with these medications shed resistant viruses by day 5 of treatment. These resistant viruses are capable of spreading infection.27

Amantadine is excreted in the urine, primarily unchanged, and requires dose adjustments for even small decreases in creatinine clearance. For this reason, care must be taken in prescribing amantadine to the elderly. In rare cases, amantadine causes elevation in the results of liver function tests. The dose of amantadine for healthy adults is 200 mg/day for 5 days. Because of drug interactions, physicians should be wary of prescribing this medication to patients already on CNS stimulants, anticholinergics, antihistamines, and certain diuretics.26,27
Rimantadine is metabolized by the liver, but a percentage is excreted unchanged in the urine. Dispensing this drug to patients with significant liver dysfunction is contraindicated. The dose for patients older than 10 years is 200 mg/day for 7 days. For patients younger than 10 years, the dose is 5 mg/kg/day to a maximum dose of 150 mg/day.26,27 (See Table 3.)
Neither M2 protein blocker is helpful for treating influenza-like illnesses, so if the level of suspicion for actual influenza is low but the patient has similar symptoms, these medications should not be used.28
The newer neuraminidase inhibitors, oseltamivir and zanamivir, also are used for prophylaxis and treatment. They are effective against both influenza A and B. They act on the neuraminidase glycoprotein and decrease the quantity of virus that can be released from the host cells. Oseltamivir is an oral medication with good bioavailability. The side effects of nausea and vomiting tend to be minimal. Starting this medication within 48 hours of symptom onset can reduce the length of symptoms by 1 day and decrease time to return to function. This medication also decreases viral shedding and, at the time of this writing, does not have the level of resistance of the M2 blockers. The dose for patients 13 years of age and older is 75 mg twice per day for 5 days. For patients with a creatinine clearance of 10-30 mL/min, the dose is reduced to once per day.26,27 Neuraminidase inhibitors also are efficacious in children by reducing the duration of symptoms. Oseltamivir may be used in children 1 year of age and older to decrease the complications of the flu and to decrease the incidence of acute otitis media by up to 50%.29
Zanamivir is an inhalation solution indicated for people age 7 years and older at a dose of 10 mg inhaled twice per day for 5 days. Zanamivir is excreted from the kidney unchanged. Instances of resistance have been noted.27 Rare bronchospasm has been associated with zanamivir.26 Both of the neuraminidase inhibitors are more costly than the M2 protein blockers.
Complications
Several illnesses can stem from infection with the influenza virus. They include primary viral pneumonia, secondary bacterial infections including pneumonia, cardiac complications, exacerbations of lung diseases such as asthma and COPD, CNS involvement, and muscle disorders. Primary viral pneumonia is more likely in the elderly and in patients with prior underlying cardiopulmonary disease, but anyone is susceptible. One day after the initial onset of viral illness, a patient with viral pneumonia rapidly will develop tachypnea, tachycardia, cyanosis, and hypotension. Rales usually are heard during examination. A chest film usually reveals nonconsolidating pulmonary infiltrates. Laboratory analyses show leukocytosis with a left shift and hypoxemia. Sputum Gram stain is negative for organisms and requires antigen testing or viral cultures. Limited studies show some advantage to treatment with amantadine or rimantadine. Treatment primarily is supportive with intravenous fluids, oxygen, and ventilatory support with positive end-expiratory pressure as necessary. Antibiotics should be administered if secondary bacterial pneumonia is suspected. Improvement begins 5-16 days after onset of pneumonia.30
Secondary bacterial pneumonia can result as a superinfection from primary viral infection. In this scenario, when the primary influenza symptoms appear to be resolving, the patient develops increasing fevers, chills, pleuritic chest pain, and productive cough. In comparison with patients with primary viral pneumonia, patients with secondary bacterial pneumonia are less tachypneic and have less cyanosis. Lung consolidation is evident on chest film. Sputum studies have a positive Gram stain, and cultures often are positive (more often so if done by bronchoalveolar lavage). The most common pathogens are Streptococcus pneumoniae, Staphylococcus aureus, Haemophilus influenza, and Moraxella catarrhalis.30 Risk factors include cardiovascular disease, COPD, pregnancy, hematologic malignancy, and advanced age.
Other bacterial infections such as cellulitis can be exacerbated by influenza viral infection. Group A streptococcus has an increased incidence in the winter, possibly associated with influenza infection. In a mouse model, the influenza virus rarely was fatal but was able to advance bacterial infections to lethality.31 Thus, it seems that neither illness is necessarily fatal but, in combination, death from a bacterial superinfection is more likely. Patients appear to have increased susceptibility to S. pneumoniae infection if they have had recent influenza infection. Studies in mice show that this increased risk may be linked to interleukin-10; when anti-IL-10 antibodies were used, the number of fatal episodes of secondary bacterial pneumonia decreased. These findings suggest a direction for research into the treatment of this type of pneumonia.32
A national survey revealed that, in patients with laboratory-confirmed influenza, 1.6% of adults and 2% of children had a secondary bacterial infection.33 Of these, 7-40% were S. aureus and 77% were methicillin-resistant S. aureus (MRSA, assumed community-acquired). S. pneumoniae accounted for 16.5-48%, and group A streptococcus was implicated in 2.1%. Hospitalization was necessary in 16% and intubation in 13%.33,34 Mortality was 14-71% in a review of influenza pneumonia.34 Given the increase in the percentage of MRSA, one should consider MRSA coverage when treating secondary bacterial pneumonia in patients at risk.34
Acute respiratory infections are a risk factor for acute myocardial infarction (AMI). Just as there is seasonality to influenza, death from AMI occurs more commonly in the winter.35 The risk of AMI is heightened for 10 days after an acute respiratory infection. After two weeks, this risk reverts to baseline.36 In a mouse study,35 it was determined that high-density lipoprotein (HDL), which normally has a protective effect on the heart, lost its anti-inflammatory properties during influenza A infection. The effect was regained 9 days after infection. The loss appeared to have been related to systemic inflammation, not the influenza virus itself, as viremia was not detected in the mice. In addition to decreased effectiveness of HDL, the acute phase reaction from an infection that causes fever also causes changes in the clotting cascade, complement activation, and, subsequently, the thrombogenic properties of the coronary arteries.35
Various infectious agents such as Chlamydia, pneumoniae, Helicobacter pylori, and cytomegalovirus may accelerate atherosclerosis.37 Chronic infection with these organisms in itself causes an inflammatory state. The concentration of C-reactive protein (CRP), which is associated with an inflammatory state, may increase during active infection; this alteration is associated with a systemic response. CRP is an independent risk factor for coronary artery disease (CAD), as atherosclerosis is at least in part inflammatory. Endothelial injury, which predisposes a person to atherosclerosis, also may be exacerbated by pathogens.38
Given these observations and associations, should people with known cardiovascular disease be considered high risk for influenza, and should they be a priority for influenza vaccine? Influenza infection is associated with an increased number of inflammatory cells in atherosclerotic plaques, fibrin deposition, and platelet aggregation, as well as endothelial dysfunction, tachycardia, and catecholamine release. A small case control study indicates that vaccination against the influenza virus may decrease recurrent MI in up to 67% of patients.39 Additionally, vaccination may reduce the incidence of out-of-hospital cardiac arrest by up to 49%.40 Further studies indicate that the incidence of stroke is associated with influenza infection, particularly among individuals younger than 75 years of age.41,42 At the First Symposium of Influenza and Cardiovascular Disease in 2003, several recommendations were made, including publicizing to the health care community the cardioprotective effects of the influenza vaccine, including the vaccine in cardiac protocols, determining which strains are most pathogenic, and increasing vaccination rates of household members of patients with pre-existing CAD.43 The PRISMA study44 revealed a 78% reduction in death and 87% reduction in acute respiratory and cardiovascular disease in vaccinated patients (18-64 years of age) with high-risk conditions compared with those not immunized.
Other known, although not common, complications of influenza infections are myocarditis and rhabdomyolysis. Diagnosing patients with viral myocarditis secondary to influenza may help predict which patients are at risk for developing idiopathic dilated cardiomyopathy later in life.45,46 A randomized controlled study to determine the prevalence of myocarditis in patients with laboratory-confirmed influenza using troponin levels concluded that myocarditis was not as prevalent as previously thought; 12% of patients had mild rhabdomyolysis. A case of rhabdomyolysis leading to compartment syndrome requiring extensive fasciotomies has been reported.47 These patients may present with what appears to be the myalgia typical of influenza, but they actually are at risk for rhabdomyolysis.47-49
Other complications, seen more often in children, include Reye's syndrome, encephalitis, febrile seizures, otitis media, and encephalopathy. Encephalopathy associated with influenza occurs in children with documented influenza A infection and manifests as fever, rapidly progressive mental status changes, and seizures.50
Prevention
Primary prevention is achieved by adequate vaccine administration to achieve herd immunity. The actual virus mutates from year to year via antigenic variations of shift and drift, which result in major and minor changes in the viral surface glycoproteins. These changes allow susceptibility to infection even among those previously exposed to the influenza virus.1 This is why new vaccines targeting the projected virus must be produced yearly. The World Health Organization (WHO) maintains global surveillance for the active strains of influenza from year to year by gathering outbreak data from 110 surveillance centers in 83 countries.51 These numbers are reported to the CDC, which in turn determines the appropriate vaccine composition for a given year using predictive modeling techniques.52 When the vaccine matches the circulating strains, it is 70-90% effective in preventing laboratory-confirmed illness in healthy adults. Reductions in severe respiratory illness and death (up to 50%) are seen among patients with comorbid conditions and patients older than 60 years.5,53 In addition to providing actual health benefits, vaccination decreases health care costs.52
The efficacy of any vaccine depends on inclusion of the viral strains in circulation as well as the age and immunocompetency of the host. The ability of the host to mount an immune response to the vaccine is paramount.52 Vaccine coverage for the 2005-2006 season will include the following strains: 1) A/California/7/2004(H3N2), 2) A/New Caledonia/20/99(H1N1), and 3) B/Shanghai/361/2002.52 Each has overlapping coverage with similar strains.
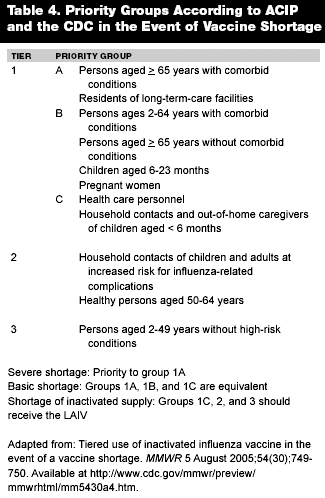
The primary groups for annual vaccination, and a tiered system for receipt when supplies run low, are listed in Table 4.52 Changes for the 2005-2006 season include extending the vaccination to any patient at increased risk for aspiration, including patients with cognitive dysfunction, spinal cord injury, seizure disorders, and other neuromuscular disorders. In addition, all health care workers are advised to receive the annual vaccine. The intranasal attenuated vaccine should be administered to all eligible populations when there is concern about the adequacy of the inactivated vaccine supply.52 The supply has run short in three of the past five years.52 Recommendations for vaccine targets and timing are published in the MMWR by the Advisory Committee on Immunization Practices (ACIP). The CDC had made vaccine prioritization for tier 1 patients to receive the inactivated influenza vaccine injection through October 24, 2005. This step was taken out of concern for adequate vaccine supply for the 2005-2006 season. As of November 10, 2005, the estimated vaccine supply exceeded last year's supply. Issues relating to inadequate supply are likely due to a perceived increased threat of a pandemic. The CDC, though, states that supply should be adequate despite one manufacturer's (Chiron) reduced production.55
The two vaccine types currently recommended in the United States are the trivalent inactivated vaccine (TIV) and the live attenuated influenza vaccine (LAIV). Both are grown initially in embryonated hens' eggs and thus may contain residual egg protein. Because of this, vaccination prior to desensitization should be avoided in patients with anaphylactic hypersensitivity to eggs.52 Vaccination also should be deferred until resolution of an acute febrile illness. Minor illnesses, upper respiratory infections, and allergic rhinitis are not contraindications. Like all interventions, vaccination can have side effects (see below), but severe reactions are rare. The Vaccine Adverse Event Reporting System is a national program to track events related to vaccination. It can be contacted through www.vaers.hhs.gov or at 800-822-7967.
Thimerosal is an antibacterial mercury compound that has been added to vaccine for decades. Because of concern about exposure to mercury, especially for infants and young children, vaccine manufacturers are in transition to a completely mercury-free vaccine product.28,52 The 2005 TIV will have only trace thimerosal, and the LAIV is thimerosal free.
The inactivated vaccine is targeted toward populations at increased risk for influenza complications. (See Table 3.)7,52 The inactivated vaccine had 38-52% effectiveness against laboratory-confirmed influenza during the 2003-2004 season, and during that season there was a suboptimal vaccine/strain match. Given these numbers, the CDC recommends continuing with vaccination even if the match is not optimal.56
Inactivated vaccine is given intramuscularly in one dose. Two doses are indicated for children younger than 9 years who have not been vaccinated before. These doses should be separated by at least 1 month.52,57 In general, vaccination should start in October, and even earlier for patients who happen to seek health care in September. In October, the focus should be on patients at high risk for complications and the contacts of these patients, e.g., health care providers. Side effects generally are localized to the site of injection.51 A minority of patients experience other side effects such as eye redness and self-limiting respiratory symptoms such as cough, wheezing, and chest tightness, which resolve in 2-48 hours after vaccination.58
The LAIV during the 2003-2004 season significantly reduced the number of severe febrile and respiratory tract illnesses in healthy adults.52 The LAIV can reduce the incidence of influenza A and B by 66-92%.59 The LAIV is indicated for healthy people between the ages of 5 and 49 years. In children 5-8 years old who have not been vaccinated before, 2 doses separated by 6-10 weeks are recommended. All others need only one dose for adequate antibody response. LAIV can shed virus for up to 7 days after the vaccine is administered. Concerns about reversion of the shed attenuated virus to a wild-type genotype with subsequent infection have not been borne out.59,60 Side effects of the LAIV include sore throat, headache, muscle aches, and cough.61
Healthy adults between the ages of 18 and 49 years may be vaccinated if the supply is adequate. A Cochrane review found that the incidence of laboratory-confirmed influenza decreased 48-70% (with both the inactivated vaccine and LIAV), but the overall incidence of clinical influenza decreased only 6%, not substantiating the need for healthy adults to be vaccinated.62
Health care workers should be targeted to receive the LAIV if they are healthy adults younger than 50 years, or the inactivated vaccine if they have any high-risk conditions or are 50 years of age or older. The CDC recommends that vaccination for this group begin in October and should be facilitated by offering no-charge vaccination at the site of work. Unfortunately, the vaccination rate among health care workers is only 40% at best.52 Vaccination among health care workers should be encouraged because it offers the benefits of lower absenteeism and reduced death rates in nursing home populations.52
Vaccination goals have not been met. Healthcare Objectives 2010 put forth a goal of 90% coverage for people older than 65 years. At a vaccination rate of 68%, this group came closest to reaching the goal during the 1999-2000 season. In 2003, the vaccination rates among high-risk adults between the ages of 18 and 64 years and healthy adults between the ages of 50 and 64 years, both target groups for vaccination, were significantly lower than the 2010 objective of 60%. Patient reports of vaccination can be accepted as verification of vaccination in clinical practice.52
Antiviral drugs can be used for prevention as well as treatment. However, they should not be substituted for vaccination but should be used as an adjunct when there is a known index case or outbreak.
Special Populations
In addition to cardiac patients, multiple other populations may be at increased risk for influenza-related complications. These include patients with pre-existing lung conditions (e.g., asthma, COPD), diabetes, human immunodeficiency virus (HIV) infection, organ transplants, and malignancies as well as residents of long-term care facilities.
A Cochrane review evaluated the importance of vaccination in patients with asthma, specifically the levels of early adverse effects caused by vaccination itself as well as the long-term protective effects. There was no significant increase in asthma exacerbations in the 2 weeks after vaccination, indicating safety for patients with asthma. Small studies did not show a difference in results regardless of type of vaccine administered.63 Of note, the evidence did not show a significant decrease in asthma exacerbations with influenza despite vaccination,63 likely because of the high incidence of other influenza-like illnesses not affected by the vaccine. A randomized controlled trial of COPD patients showed that vaccination reduced the incidence of respiratory infections related to influenza; therefore, vaccination of these patients was recommended. The study was too small to determine the effect on hospital admission rates or mortality.64 The importance of the vaccine lies in that a large proportion of COPD exacerbations are caused by viral or bacterial infection. Preventing viral infections alone may decrease COPD events; in addition, viral infection may make a patient more susceptible to bacterial infection and subsequent morbidity.64
Diabetics have baseline immune dysfunction but are able to mount an appropriate response to a vaccine. Vaccination in this group decreases the numbers of hospital admissions for influenza and its complications.65
The efficacy of the influenza vaccine in patients with HIV is similar to that in immunocompetent adults. Varying effects of the vaccine on the HIV viral load have been reported.66 Patients with HIV and AIDS should be targeted for vaccination because they are at increased risk of death from influenza and pneumonia.67
The influenza vaccine is recommended yearly for people who have received organ transplants. There is increased morbidity among these patients if they have influenza infection. Some patients with transplants have a poor antibody response to the vaccine, but, when infection does occur, vaccinated patients tend to have a less severe course than the unimmunized. The greatest risk is to patients younger than 1 year of age, those within 3 months of a solid organ transplant, and those who contract influenza infection while undergoing therapy for rejection. It is unknown if there are drug interactions between antivirals and the commonly used transplant medications. Nonetheless, viral prophylaxis is recommended for patients with solid organ transplant for post-exposure prophylaxis. In addition, community-acquired respiratory viruses are associated with allograft rejection.68
Despite, and because of, the immunocompromised state of the patient with a hematologic malignancy, vaccination for influenza is recommended. Some protection, though limited, is conveyed.69
Patients in long-term care facilities, in addition to having a myriad of comorbidities, live in close contact with one another. The risk of spread from an index case is quite high. For outbreaks, the CDC recommends prophylaxis for residents and staff. If the isolate is influenza A, rimantadine is recommended over amantadine because of its better side effect profile, especially in the elderly, who may be prone to more of the drug's CNS effects. If this chemoprophylaxis does not appear to be effective or if the isolate is influenza B, the use of oseltamivir for at least 2 weeks is recommended, or for 1 week after the last documented case.70
Avian Influenza
The avian influenza virus first was isolated in 1961 from birds in South Africa. The avian influenza virus is carried in wild bird intestines but can cause illness in domesticated birds. Birds carry all influenza A subtypes. This virus is very contagious among birds, and there is a silent reservoir in ducks. It is spread via shed virus in the saliva, nasal secretions, and feces. Symptoms in humans include the expected characteristics of influenza as well as eye infections, pneumonia, and adult respiratory distress syndrome (ARDS).71 Avian influenza carries a high mortality rate. Disease can occur without respiratory symptoms, so many cases may be missed. H5 and H7 subtypes of avian influenza A viruses are considered either highly pathogenic or low pathogenic. When the highly pathogenic strains infect poultry, mortality can be 90% to 100%; the low pathogenic viruses cause minimal or no disease. It is unknown how these levels of pathogenicity affect humans.72 The strains circulating at this time are more pathogenic than earlier strains of the virus.73 Avian strains usually do not infect humans well because HA proteins that are avian-adapted do not bind well with human receptors. It previously was thought that the virus had to go through a pig intermediary host. Hosts of H5N1 are expanding to tigers, cats, and swine.74
In 1997, the first bird-to-human viral transmission of the influenza virus (H5N1) was documented in Hong Kong; this transmission caused 18 cases of severe illness and 6 deaths. Prior to 1997, H5N1 influenza was not known to circulate among humans; therefore, this outbreak caused public health concerns worldwide.75 By late 2003, H5N1 outbreaks in birds had occurred in eight countries: Cambodia, China, Indonesia, Japan, Laos, South Korea, Thailand, and Vietnam. More than 100 million birds were killed by disease or were exterminated in hopes of stemming the epidemic. Human cases of the avian flu A(H5N1) appeared in Thailand and Vietnam in January 2004. At that time, the WHO declared pandemic preparedness phase 0, level 2, indicating no evidence of sustained human-to-human spread.57 These cases were caused by the same strain that caused the 2003 poultry outbreaks in eight Asian nations.75 It initially was thought that the likelihood of avian flu expanding to epidemic proportions was unlikely, given the virus's improbable ability to achieve human-to-human spread, but there have been two reported cases of this type of transmission. Both occurred among family members providing unprotected care to an index patient with H5N1.76 As of November 9, 2005, there were 125 confirmed human cases of avian influenza A(H5N1) in Asia that were reported to the World Health Organization; half of those people died,77 most of them previously healthy children and young adults.
Other types of avian flu A in humans have been implicated and confirmed in the avian flu crisis since 1997: H9N2 and H7N2 in Asia; H7N7 in the Netherlands, which was mostly conjunctivitis; and H7N2 and H7N3 in New York, Virginia, and Canada.78 In North America, outbreaks in poultry have occurred in Texas, Maryland, Pennsylvania, Delaware, and New Jersey.79 Human cases have not been identified in these areas, but the detection of the influenza virus in these birds lends gravity to the situation that the United States is only a mutation away from a human-avian influenza epidemic.
Genetic reassortment between avian and human influenza viruses has not yet occurred, but if it should happen, it will facilitate human-to-human transmission. Confirmation of infection and serotype is important for tracking the disease and its epidemiologic progression. Rapid antigen testing can detect H5N1, but with limited sensitivity compared with PCR or viral culture. If there is a high level of suspicion of avian influenza based on clinical presentation and epidemiologic data, specimens should be sent to the CDC if they test positive for influenza A or if no testing is available. Arrangements should be made by state health departments, who contact the CDC Emergency Operations Center.80 Although the avian influenza virus from Asia tested resistant to the M2 protein blockers, it appears sensitive to oseltamivir,74,80,81 leading to possible prophylaxis or treatment in humans. There has been a report of an oseltamivir resistant H5N1 virus isolated from a Vietnamese girl.82 Sensitivity of the avian influenza virus to zanamivir is unknown at this time. Given the possibility of epidemic avian flu as well as the uncertainty of effective treatment, vaccine prevention would be ideal. Reference viruses have been produced to aid manufacturers in developing a vaccine.73 National and international efforts have begun; vaccine trials started in April 2005 through the National Institute of Allergy and Infectious Diseases, and early vaccines show promise for effectiveness in humans. The federal government has earmarked significant sums of money for the development of this vaccine as well as for pandemic surveillance and antiviral acquisition. The FDA has formed a Rapid Response Team to ensure adequate acquisition of appropriate antiviral drugs, such as oseltamivir, as well has other drugs still being tested that may be available under Emergency Use Authorization. The goal of this team also includes monitoring for counterfeit drugs that may become prevalent as the avian flu becomes a more significant public health issue.83
Testing for the avian flu is indicated in certain patients. Hospitalized patients should be tested if they have pneumonia on chest film, if they have ARDS or another severe respiratory illness without documented cause, and if they have traveled to an area with documented H5N1 avian influenza in birds or humans. Outpatients should be tested if they have a temperature greater than 38°C; a cough, sore throat, or shortness of breath; and a history of contact with a human or bird with documented or suspected H5N1 within 10 days of symptom onset.71
Emergency Department Management
During the initial stages of widespread influenza infection, there is increased emergency department (ED) utilization, which is associated with increased ED length of stay, number of patients admitted to the ED, numbers of patients who leave without being seen, diversion of emergency medical services (EMS), as well as increased overall patient census.84
The American College of Emergency Physicians (ACEP) has issued a statement that describes how to decrease the impact of an influenza outbreak on a community. This statement recommends that all health care providers, including EMS personnel, be vaccinated. There must be protocols for the rapid identification of patients with possible influenza coming to the ED and an efficient mechanism for isolating them. Boarding patients in the ED can be detrimental; admitted patients need to be moved out of the department regardless of bed availability to allow more room for patients in the ED and prevent person-to-person transmission. During a severe outbreak, elective admissions should be suspended.85 Airborne precautions should be used when treating patients with known or suspected influenza to decrease the transmission rate within the ED.
Isolation precautions for possible avian influenza are the same as those for SARS, which include standard and contact precautions, use of eye protection when within 3 feet of an infected patient, as well as airborne precautions (negative pressure rooms, with 6 to 12 air changes per hour). An N-95 filtering mask, as approved by the National Institute for Occupational Safety and Health (NIOSH), should be worn by all persons who enter the patient's room.86
Because the ED has become a place where many people receive their only medical care, it may be a place where vaccination and education systems can be helpful. High-risk patients frequent the ED because of their conditions. More than half of these patients do not get vaccinated.85 Such patients presenting to the ED are prime targets to be offered the vaccine. Most emergency physicians are willing to dispense the vaccine, and doing so may increase vaccination in high-risk groups by one-third.87
Patients come to the ED when they are feeling their worst, when they have no other place to go, and when they want relief. Emergency physicians have the ability to provide analgesia, education, and possible prevention strategies for the spread of the influenza virus, including isolation, medications, and vaccination.
References
1. Sarubbi FA. Influenza: A historical perspective. South Med J 2003;96:735-736.
2. Palese P. Influenza: Old and new threats. Nature Medicine Supplement 2004;10:S82-S87.
3. Update: Influenza activity-United States, 2004-05 season. 2005;54:328-331. Available at www.cdc.gov/mmwr/preview/mmwrhtml/mm5413a2.htm. Accessed 9/16/2005.
4. Thompson WW, Shay DK, Weintraub E, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA 2003;289:179-186.
5. World Health Organization. Influenza vaccines: WHO position paper. Weekly Epidemiological Record 2002;220;77(28):229-240. Available at www.who.int/docstore/wer/pdf/2002/wer7728.pdf. Accessed 9/16/2005.
6. Thomson WW, Shay DK, Weintraub E. Influenza-associated hospitalizations in the United States. JAMA 2004;292:1333-1340.
7. Glezen WP. The changing epidemiology of respiratory syncytial virus and influenza: impetus for new control measures. Pediatr Infect Dis J 2004;23:S202-S206.
8. Irwin DE, Weatherby LB, Huang WY, et al. Impact of patient characteristics on the risk of influenza/ILI-related complications. BMC Health Services Research 2001;1:8. Available at http://www.biomedcentral.com/1472-6963/1/8.
9. Moorman JP. Viral characteristics of influenza. South Med J 2003;96:758-761.
10. Tamura S, Kurata T. Defense mechanisms against influenza virus infection in the respiratory tract mucosa. Jpn J Infect Dis 2004;57:236-247.
11. Bolyard EA, Tablan OC, Williams WW, et al. Guideline for infection control in health care personnel, 1998. AJIC: American Journal of Infection Control 1998;26:289-354.
12. Call SA, Vollenweider MA, Hornung CA, et al. Does this patient have influenza? JAMA 2005;283:987-997.
13. Shorman M, Moorman JP. Clinical manifestations and diagnosis of influenza. South Med J 2003;96:737-739.
14. Ebell MH, White LL, Casault T. A systematic review of the history and physical examination to diagnose influenza. J Am Board Fam Pract 2004;17:1-5.
15. Zambon M, Hays J, Webster A, et al. Diagnosis of influenza in the community: Relationship of clinical diagnosis to confirmed virological, serologic, or molecular detection of influenza. Arch Intern Med 2001;161:2116-2122.
16. Ruest A, Michaud S, Deslandes S, et al. Comparison of the Directigen FluA+B test, the QuickVue influenza test, and clinical case definition to viral culture and reverse transcription-PCR for rapid diagnosis of influenza virus infection. J Clin Microbiol 2003;41:3487-3493.
17. Bonner AB, Monroe KW, Talley LI, et al. Impact of the rapid diagnosis of influenza on physician decision-making and patient management in the pediatric emergency department: Results of a randomized, prospective, controlled trial. Pediatrics 2003;112:363-367.
18. Templeton KE, Scheltinga SA, Beersma MFC, et al. Rapid and sensitive method using multiplex real-time PCR for diagnosis of infections by influenza A and influenza B viruses, respiratory syncytial virus, and parainfluenza viruses 1, 2, 3, and 4. J Clin Microbiol 2004;42(4):1564-1569.
19. Esposito S, Marchisio P, Morelli P, et al. Effect of a rapid influenza diagnosis. Arch Dis Child 2003;88:525-526.
20. Uyeki TM. Influenza diagnosis and treatment in children: A review of studies on clinically useful tests and antiviral treatment for influenza. Pediatr Infect Dis J 2003;22:164-177.
21. CDC. Influenza: Laboratory diagnostic procedures for influenza. MMWR 2005;54(No. RR08). Available at www.cdc.gov/flu/professionals/labdiagnosis.htm. Accessed on September 16, 2005.
22. Pachucki CT, Khurshid MA, Nawrocki J. Utility of reverse transcriptase PCR for rapid diagnosis of influenza virus infection and detection of amantadine-resistant influenza A virus isolates. J Clin Microbiol 2004;42:2796-2798.
23. Gandey A. Is it flu or SARS? MDs gear up for a difficult winter. CMAJ 2003;169:821.
24. Cinti SK, Saravolatz L, Nafziger D, et al. Differentiating inhalational anthrax from other influenzalike illnesses in the setting of a national or regional anthrax outbreak. Arch Intern Med 2004;164:674-676.
25. Fine AM, Wong JB, Fraser HS, et al. Is it influenza or anthrax? A decision analytic approach to the treatment of patients with influenza-like illnesses. Ann Emerg Med 2004; 43:318-328.
26. WHO Guidelines on the Use of Vaccines and Antivirals during Influenza Pandemic. 2004.
27. Myers JW. Influenza therapy. South Med J 2003;96:744-750.
28. Moylett EH, Hanson IC. Mechanistic actions of the risks and adverse events associated with vaccine administration. J Allergy Clin Immunol 2004;114:1010-1020.
29. Matheson NJ, Symmonds-Abrahams M, Sheikh A, et al. Neuraminidase inhibitors for preventing and treating influenza in children. The Cochrane Database of Systematic Reviews 2003, Issue 3:CD002744.DOI: 10.1002/14651858.CD002744.
30. Khater F, Moorman JP. Complications of influenza. South Med J 2003;96:740-743.
31. Okamoto S, Kawabata S, Nakagawa I, et al. Influenza A virus-infected hosts boost an invasive type of Streptococcus pyogenes infection in mice. J Virol 2003;77:4104-4112.
32. van der Sluijs KF, van Elden LJR, Nijhuis M, et al. IL-10 is an important mediator of the enhanced susceptibility to pneumococcal pneumonia after influenza infection. J Immunol 2004;172:7603-7609.
33. Podewils LJ, Liedtke LA, McDonald LC, et al. A national survey of severe influenza-associated complications among children and adults, 2003-2004. Clin Infect Dis 2005;40:1693-1696.
34. Oliveira EC, Marik PE, Colice G. Influenza pneumonia: a descriptive study. Chest 2001;119:1717-1723.
35. Van Lenten BJ, Wagner AC, Nayak DP, et al. High-density lipoprotein loses its anti-inflammatory properties during acute influenza A infection. Circulation 2001;103:2283-2288.
36. Meier CR, Jick SS, Derby LE, et al. Acute respiratory-tract infections and the risk of first-time myocardial infarction. Lancet 1998;351:1467-1471.
37. Danesh J, Collins R, Peto R. Chronic infections and coronary heart disease: is there a link? Lancet 1997;350:430-436.
38. Prasa A, Zhu J, Halcox JPJ, et al. Predisposition to atherosclerosis by infections: roles of endothelial dysfunction. Circulation 2002;106:184-190.
39. Naghavi M, Barlas Z, Siadaty S, et al. Association of influenza vaccination and reduced risk of recurrent myocardial infarction. Circulation 2000;102:3039-3045.
40. Siscovick DS, Raghunathan TE, Lin D, et al. Influenza vaccination and the risk of primary cardiac arrest. Am J Epidemiol 2000;152:674-677.
41. Lavallee P, Perchaud V, Gautier-Bertrand M, et al. Association between influenza vaccination and reduced risk of brain infarction. Stroke 2002;33:513-518.
42. Grau AJ, Fischer B, Barth C, et al. Influenza vaccination is associated with a reduced risk of stroke. Stroke 2005;36:1501-1506.
43. Madjid M, Aboshady I, Awan I, et al. Influenza and cardiovascular disease: Is there a causal relationship? Tex Heart Inst J 2004;31:4-13.
44. Hak E, Buskens E, van Essen GA, et al. Clinical effectiveness of influenza vaccination in persons younger than 65 years with high-risk medical conditions: The PRISMA Study. Arch Intern Med 2005;165:274-280.
45. Greaves K, Oxford JS, Price CP, et al. The prevalence of myocarditis and skeletal muscle injury during acute viral infection in adults: Measurement of cardiac troponins I and T in 152 patients with acute influenza infection. Arch Intern Med 2003;163:165-168.
46. Kuhl U, Pauschinger M, Noutsias M, et al. High prevalence of viral genomes and multiple viral infections in the myocardium of adults with "idiopathic" left ventricular dysfunction. Circulation 2005;111:887-893.
47. Swaringen JC, Seiler JG, Bruce RW. Influenza A induced rhabdomyolysis resulting in extensive compartment syndrome. Clin Orthop Related Res 2000;375:243-249.
48. McDonnell GV. A bad dose of ‘flu. Postgrad Med J 2002; 78:373.
49. Morton SE, Mathai M, Byrd, RP, et al. Influenza A pneumonia with rhabdomyolysis. South Med J 2001;94(1):67-69.
50. Weitkamp JH, Spring MD, Brogan T, et al. Influenza A virus-associated acute necrotizing encephalopathy in the United States. Pediatr Infect Dis J 2004;23:259-263.
51. Langley JM, Faughnan ME. Prevention of influenza in the general population. CMAJ 2004;171:1213-1222.
52. CDC. Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practice (ACIP). MMWR 2005;54(No RR08).
53. Govaert TME, Thijs CTMCN, Masurel N, et al. The efficacy of influenza vaccination in elderly individuals. JAMA 1994; 272:1661-1665.
54 Tiered use of inactivated influenza vaccine in the event of a vaccine shortage. MMWR 5 August 2005;54(30);749-750. Available at http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5430a4.htm. Accessed 10/5/2005.
55. Centers for Disease Control and Prevention. Questions and Answers: Vaccine supply and prioritization recommendations for the U.S. 2005-06 influenza season. Centers for Disease Control and Prevention web site. Available at www.cdc.gov/flu/about/qa/0506supply.htm. Accessed 11/12/2005.
56. Assessment of the effectiveness of the 2003-04 influenza vaccine among children and adults-Colorado, 2003. MMWR 13 August 2005; 53(31);707-710. Available at www.cdc.gov/mmwr/preview/mmwrhtml/mm5331a1.htm. Accessed 10/5/2005.
57. Recommended composition of influenza virus vaccines for use in the 2005-2006 influenza season. Weekly Epidemiological Record 25 February 2005;80(8):65-76. Available at www.who.int/wer/2005/wer8008.pdf. Accessed 10/4/2005.
58. Influenza vaccines. Weekly Epidemiological Record 12 July 2002;77(28):229-240. Available at http://www.who.int/docstore/wer/pdf/2002/wer7728.pdf. Accessed 10/5/2005.
59. Greenberg HB, Peidra PA. Immunization against viral respiratory disease: A review. Pediatr Infect Dis J 2004;23:S254-S261.
60. Talbot TR, Crocker DD, Peters J, et al. Duration of virus shedding after trivalent intranasal live attenuated influenza vaccination in adults. Infect Control Hosp Epidemiol 2005;26:494-500.
61. Jefferson T, Deeks JJ, Demicheli V, et al. Amantadine and rimantadine for preventing and treating influenza A in adults. The Cochrane Database of Systematic Reviews 2004, Issue 3. Art. No. CD001169.pub2. DOI: 10.1002/14651858.CD001169.pub.2.
62. Demicheli V, Rivetti D, Deeks JJ, et al. Vaccines for preventing influenza in healthy adults. The Cochrane Database of Systematic Reviews 2004, Issue 3. Art. No.: CD001269.pub2. DOI: 10.1002/14651858.CD001269.pub2.
63. Cates CJ, Jefferson TO, Bara AI, et al. Vaccines for preventing influenza in people with asthma. The Cochrane Database of Systematic Reviews 2003, Issue 4. Art. No.: CD000364.pub2. DOI: 10.1002/14651858.CD000364.pub2.
64. Wongsurakiat P, Maranetra KN, Wasi C, et al. Acute respiratory illness in patients with COPD and the effectiveness of influenza vaccination. Chest 2004;125:2011-2020.
65. Smith SA, Poland GA, American Diabetes Association. Influenza and pneumococcal immunization in diabetes. Diabetes Care 2004;27:S111-S113.
66. Tasker SA, Treanor JJ, Paxton WB, et al. Efficacy of influenza vaccination in HIV-infected persons: A randomized, double-blind, placebo-controlled trial. Ann Intern Med 1999;131:430-433.
67. Lin JC, Nichol KL. Excess mortality due to pneumonia or influenza during influenza seasons among persons with acquired immunodeficiency syndrome (original investigation). Arch Intern Med 2001;161:441-446.
68. Slifkin M, Doron S, Snydman DR. Viral prophylaxis in organ transplant patients. Drugs 2004;64:2763-2792.
69. Ljungman P, Nahi H, Linde A. Vaccination of patients with haematological malignancies with one or two doses of influenza vaccine: a randomized study. Br J Haematol 2005;130:96-98.
70. Dumyati G, Falsey AR. Antivirals for influenza: What is their role in the older patient. Drugs Aging 2002;19:777-786.
71. Past updates from the clinician registry listserv: Update sent August 14, 2004: Update on avian influenza A (H5N1). Centers for Disease Control and Prevention, 2004. Available at www.bt.cdc.gov/coca/updates/2004aug13-h5n1.asp. Accessed 10/5/2005.
72. CDC. Influenza viruses. Centers for Disease Control, Available at www.cdc.gov/flu/avian/gen-info/flu-viruses.htm. Accessed 10/7/2005.
73. CDC. Recent avian influenza outbreaks in Asia. Centers for Disease Control and Prevention, August 5, 2005. Available at www.cdc.gov/flu/avian/outbreaks/asia.htm. Accessed 10/5/2005.
74. Hien TT, de Jong M, Farrar J. Avian influenza-a challenge to global health care structures. N Engl J Med 2004;351: 2363-2365.
75. World Health Organization: Strengthening pandemic influenza preparedness and response: Report by the Secretariat 7 April 2005. Available at www.wpro.who.int/NR/rdonlyres/4C7B0575-872B-4E9C-A7C7-8BFF014F10A1/0/A58_13en.pdf. Accessed 11/14/2005.
76. Ungchusak K, Auewarakul P, Dowell SF, et al. Probable person-to-person transmission of avian influenza A (H5N1). N Engl J Med 2005;352:333-340.
77. World Health Organization. Cumulative number of confirmed human cases of avian influenza A/(H5N1) reported to WHO. World Health Organization web site. Available at www.who.int/csr/disease/avian_influenza/country/cases_table_2005_11_09/en/index.html. Accessed 11/12/2005.
78. CDC. Avian influenza infection in humans. Centers for Disease Control and Prevention, May 24, 2005. Available at www.cdc.gov/flu/avian/gen-info/avian-flu-humans.htm. Accessed 10/5/2005.
79. CDC. Outbreaks in North America: Outbreaks in North America with transmission to humans. Centers for Disease Control and Prevention. Available at www.cdc.gov/flu/avian/outbreaks/us.htm. Accessed 10/5/2005.
80. Hammel JM, Chiang W. Update on emerging infections: news from the Centers for Disease Control and Prevention. Ann Emerg Med 2005;45:88-90.
81. Yen HL, Monto AS, Webster RG, et al. Virulence may determine the necessary duration and dosage of oseltamivir treatment for highly pathogenic A/Vietnam/1203/04 influenza virus in mice. J Infect Dis 2005;192:665-672.
82. Le QM, Kiso M, Someya K, et al. Isolation of drug-resistant H5N1 virus. Nature 2005;437:1108.
83. FDA announces rapid response team to combat pandemic (avian) flu. U.S. Food and Drug Administration. Available at http://www.fda.gov/bbs/topics/NEWS/2005/NEW01248.html. Accessed on November 1, 2005.
84. Silka PA, Geiderman JM, Goldberg JB, et al. Demand on ED resources during periods of widespread influenza activity. Am J Emerg Med 2003;21:534-539.
85. Policy statements: Emergency department utilization during outbreaks of influenza. Ann Emerg Med 2005;45:686.
86. CDC. Update on avian influenza A: interim recommendations: infection control precautions for influenza A (H5N1). Centers for Disease Control and Prevention, February 4, 2005. Available at www.cdc.gov/flu/avian/professional/han020405.htm. Accessed 10/5/2005.
87. Kapur AK, Tenenbein M. Vaccination of emergency department patients at high risk for influenza. Acad Emerg Med 2000;7:354-358.