Authors:
Chadd E. Nesbit, MD, PhD, FACEP, Attending Physician, Emergency Medicine, Adjunct Assistant Professor, Temple University, Assistant Medical Director, Life Flight, Allegheny General Hospital, Pittsburgh, PA
Margaret C. Powers, MD, Emergency Medicine and Internal Medicine Residency, Allegheny General Hospital, Pittsburgh, PA
Peer Reviewer:
Jay Menaker, MD, FACEP, Associate Professor, Department of Surgery/Emergency Medicine, University of Maryland School of Medicine, R Adams Cowley Shock Trauma Center, Baltimore, MD
Executive Summary
- In the United States, alcohol intoxication associated with prolonged muscle compression and/or seizures is the most common cause of traumatic rhabdomyolysis.
- Discolored urine (pink, red, brown, or even black) can be an early sign of rhabdomyolysis. Later signs include malaise, fever, tachycardia, nausea, and vomiting.
- Aoki et al analyzed predictors for developing crush syndrome in victims of the Kobe earthquake and found that tachycardia greater than 120 beats/min, delayed rescue greater than 3 hours, and abnormal urine color were most predictive.
- Indications for dialysis include: hyperkalemia resistant to medical therapy, metabolic acidosis resistant to medical therapy, uremia and its complications (i.e., pericarditis), or volume overload.
- Creatine kinase (CK) level is the most sensitive marker for rhabdomyolysis, and it is also a predictor for the development of renal failure.
- Oda et al found that 84% of patients with a peak CK greater than 75,000 were very likely to develop acute renal failure and require hemodialysis.
- In crush victims with acute closed compartment syndrome, fasciotomy should not be routine, and providers should be aware of the risk of infection.
Crush syndrome is a serious, life-threatening condition that can develop after a traumatic compressive injury. Hallmarks of this condition include acute renal failure, hypotension, and metabolic abnormalities such as metabolic acidosis and hyperkalemia. Clinicians should have a high degree of suspicion for this syndrome in patients who present to the hospital after suffering a crush injury, as it carries a high morbidity and mortality. Prevention of potential complications may be achieved through early, aggressive fluid resuscitation, often prior to or during extrication. The authors will review the pathophysiology, clinical presentation, and management of crush syndrome.
— Ann M. Dietrich, MD, Editor
Definitions
Crush injury is a direct compressive injury to the extremities or other part of the body that results in local injury to muscles and nerves. Crush syndrome includes a localized crush injury along with systemic manifestations of muscle cell damage such as shock, rhabdomyolysis, and renal failure. Crush syndrome may also be called traumatic rhabdomyolysis.
Epidemiology
Crush syndrome was first recognized in the early 20th century. There were reports of muscle injury and renal failure in patients during the Messina earthquake in 1909 and in German soldiers buried alive during World War I.1 The first description of crush syndrome in the English language was written in 1941 by Bywaters, who described the development of dark urine, renal failure, shock, and, ultimately, death in patients who sustained crush injuries during air raids in London during World War II.2 Since this time, crush syndrome has been reported in victims of warfare and natural disasters, especially earthquakes. The incidence of crush syndrome in earthquake victims ranges from 11-14%.3,4,5 Mortality in these patients is high, around 13-14%.3,5 Apart from direct trauma, crush syndrome is the most common cause of death in earthquake victims.6 The specific cause of death in these victims includes hypovolemia, hyperkalemia, and circulatory failure.7 Smith et al characterized the main causes of death in crush victims based on time after injury. (See Table 1.) The common causes of immediate death include severe head injury, traumatic asphyxia, and intrathoracic or intra-abdominal injury. Causes of early death include hyperkalemia and hypovolemia. Finally, causes of late death include renal failure, coagulopathy or hemorrhage, and sepsis.8
Table 1. Causes of Death in Crush Victims
Causes of immediate death
- Severe head injury
- Traumatic asphyxia
- Intrathoracic or intra-abdominal injuries
Causes of early death
- Hyperkalemia
- Hypovolemia
Causes of late death
- Renal failure
- Coagulopathy
- Hemorrhage
- Sepsis
Site of Injury. In victims of the 1995 Kobe earthquake with crush syndrome, most patients had lower extremity injury (73.7%), followed by upper extremity injury (9.7%) and trunk injury (8.6%); however, mortality was highest in patients with trunk injuries (52%). Only 31% had associated fractures. The most common fractures were of the pelvis (9.9%) and extremities (7.8%). Abdominal injury was rare (4.3%) but had a high mortality (50%).7
Time Under the Rubble. Time under the rubble has not been a reliable predictor of mortality. In the 1995 Kobe, Japan, earthquake, time trapped had no correlation with severity of crush syndrome. A subset of patients with serum creatinine kinase (CK) levels greater than 75,000, who had a higher incidence of renal failure and mortality, spent less time under the rubble than patients with CK levels less than 75,000.7 In the 1999 Marmara earthquake, victims who were trapped longer had lower rates of mortality and need for dialysis.9 In contrast, in the 2003 Bam earthquake, victims who spent more time under the rubble were more likely to develop acute renal failure.10 However, victims of the Bam earthquake spent less time under the rubble compared to victims of the Marmara and Japan earthquakes (average time to rescue less than 4 hours versus 11.7 hours and 9.0 hours, respectively).7,9 This suggests that in major catastrophes, victims with the most serious injury may die before extrication, while the less-injured victims are able to endure longer periods under the rubble.
Etiology
Most crush injuries, particularly those described in the literature, result from natural disasters such as earthquakes, warfare, and building/structural collapse. Crush injury can also be seen in patients who are run over by vehicles and in patients with altered level of consciousness (particularly those with overdose, intoxication, or stroke) who crush part of their body with their own weight for a prolonged period of time.8 In the United States, alcohol intoxication associated with prolonged muscle compression and/or seizures is the most common cause of traumatic rhabdomyolysis.11
Pathophysiology
Bywaters’ investigation of crush syndrome in 1941 included a post-mortem microscopic examination of the kidneys in which he discovered debris and casts of a "brownish pigment" that he later identified as myoglobin in 1943.2 While myoglobin is one of the main agents of destruction in crush syndrome, there are several different mechanisms of damage to the myocyte. These are summarized in Table 2.
Table 2. Mechanisms of Injury in Crush Syndrome
Action |
Results |
|
Disruption of the sarcolemma membrane/destruction of Na-K-ATPase pumps |
|
|
Reperfusion injury |
|
|
Release of muscle contents |
|
|
Compartment syndrome |
|
Crush injury disrupts the sarcolemma membrane and its sodium-potassium-ATPase pumps. The myocyte has a negatively charged and hyperosmotic cytoplasm, relative to the extracellular fluid. Mechanical stress causes damage to the sarcolemma membrane, resulting in disruption of the cell’s permeability and inability to effectively pump out sodium. Sodium, calcium, and fluid are suddenly able to enter the myocyte, causing it to swell. This redistribution of fluid can lead to third spacing and hypovolemic shock.1 Excess intracellular calcium leads to persistent muscle contraction, depletion of cellular energy, and cell death.12 Calcium also triggers several molecular cascades, including the production of free oxygen radicals. Free radicals activate neutrophils, releasing proteases and more oxygen radicals, creating a cascade of myocyte destruction.13
As myocytes begin to die, toxic contents build up within the cell. However, blood vessels are crushed along with muscle, preventing circulation to the necrotic tissue and, thus, blocking the release of these substances. Once the crushing force is removed from the body, blood is suddenly able to circulate to and from the muscle. This process is known as reperfusion syndrome, and can be very destructive. Toxins produced during myocyte necrosis are suddenly able to circulate to the kidneys and rest of the body. Leukocytes migrate into the muscle and promote free radical formation now that oxygen is again supplied to the tissue. Free radicals subsequently damage the cell membrane via lipid peroxidation and cause cell lysis.11 Fluid diffuses rapidly into myocytes, causing massive edema and third spacing. This sudden, massive fluid shift, and the release of potassium into circulation, can cause cardiac arrest; in fact, 20% of earthquake deaths occur right after extrication.14 Prevention of this phenomenon by aggressive fluid resuscitation prior to extrication is, therefore, very important in management of crush injury.
Skeletal muscle contains a high concentration of myoglobin, an oxygen-binding protein. Myoglobin itself is nephrotoxic and causes acute tubular necrosis, likely via free radical-mediated injury catalyzed by the iron moiety of the protein. Additionally, myoglobin reacts with Tamm-Horsfall proteins in the renal tubules to form casts, which subsequently obstruct the renal tubules, just as Bywaters recognized. Both free radical and cast formation are enhanced in an acidic, hypovolemic environment, which is often present in crush injury.13 Hypovolemia and obstruction of renal tubules also leads to renal vasoconstriction, which worsens renal ischemia.15
Other myocyte contents contribute to development of metabolic derangements, including hyperkalemia, metabolic acidosis, hyperphosphatemia, and hypocalcemia. Dying myocytes release organic acids (such as sulfonic acid and phosphoric acid), lactic acid, and nucleic acids, which are converted into uric acid. As the kidneys begin to fail, these acids cannot be eliminated and build up in the bloodstream. Acidosis can worsen hyperkalemia and promote myoglobin precipitation in the renal tubules.16 As discussed above, there is an influx of calcium into the myocyte. Excess intracellular calcium can precipitate in the muscle, producing hypocalcemia.17 However, clinicians should be wary of replacing calcium in crush victims, as calcium salts are subsequently released in the recovery phase and cause hypercalcemia.18 Myocytes also release tissue thromboplastin, which can promote disseminated intravascular coagulation (DIC), another common complication of crush syndrome.11
Crush injury by itself causes significant cellular edema and third spacing due to damage of sodium-potassium pumps. This edema can lead to elevated intramuscular pressure, especially within a rigid anatomical compartment such as the calf or forearm. Excessive swelling of the myocytes leads to compression of blood vessels within the compartment, worsening tissue perfusion and producing muscle and capillary bed ischemia. This process, known as compartment syndrome, furthers tissue death and edema.19
Understanding the pathophysiology of crush syndrome translates into appropriate treatment of the condition. Treatment of crush syndrome focuses on preventing large fluid shifts via adequate resuscitation, controlling the metabolic abnormalities that result from expulsion of myocyte contents into circulation, prevention of myoglobin precipitation in the renal tubules, and recognition of compartment syndrome.
Clinical Features
Clinical features are not especially helpful in diagnosing crush syndrome, as they may be highly variable and non-specific. Bartal et al found that the most common clinical signs in patients with crush syndrome in the 2010 Haiti earthquake were abdominal pain, generalized weakness, and muscular pain independent of the crushed area.4 Discolored urine (pink, red, brown, or even black) can be an early sign of rhabdomyolysis. Later signs include malaise, fever, tachycardia, nausea, and vomiting.20 Patients will often have some evidence of extremity trauma, such as skin injury, erythema, ecchymosis, abrasion, or swelling. The limb may not be painful, although it may be numb. Direct arterial injury is rare in crush syndrome, so pulses are usually palpable.1 As time progresses, patients may develop severe pain in the limb, weakness, paresthesia, paralysis, pallor, and loss of pulses due to compartment syndrome. Some patients may have little or no signs of external injury. In fact, the majority of patients with crush syndrome do not have serious musculoskeletal injuries.7 Clinicians should perform a thorough physical examination and history before releasing patients with a mechanism concerning for crush injury.
Key features that clinicians should look for include: traumatic limb injury, signs of compartment syndrome, oligoanuria, or hypovolemia. Aoki et al analyzed predictors for developing crush syndrome in victims of the Kobe earthquake and found that tachycardia greater than 120 beats/min, delayed rescue greater than 3 hours, and abnormal urine color were most predictive.21
Diagnostic Studies
In crush victims, the serum potassium level should be obtained as soon as possible. Hyperkalemia was present in 15.9% of crush syndrome victims of the Wenchuan earthquake (although this figure only includes admitted patients).22 Victims can develop hyperkalemia prior to the development of renal failure and may go into cardiac arrest in the field. Portable EKG devices can be used to indirectly detect hyperkalemia. In the 2010 Haiti earthquake, point-of-care devices (i-STAT) were used to quickly measure electrolytes and creatinine.23 Responders should be prepared to treat hyperkalemia, if present, with calcium gluconate, insulin and dextrose, beta-agonists, and sodium bicarbonate. Severe hyperkalemia that is not responsive to medical treatment is an indication for urgent hemodialysis. Sever et al found that hyperkalemia was the most significant predictor of the need for dialysis in crush syndrome victims of the Marmara earthquake who were admitted in the first 3 days. Victims who presented early tended to have a higher potassium level (average 5.4 ± 1.3 mEq/l) than those who presented later, although 4.7% of late-presenting patients had severe hyperkalemia.24 Therefore, clinicians must always be vigilant about the possibility of hyperkalemia in crush victims, regardless of when they present for evaluation.
Upon arrival to the hospital, patients should have a complete blood count, basic metabolic panel, creatine kinase level, venous (or arterial) blood gas, and urinalysis. These results will indicate blood loss and need for transfusion, electrolyte abnormalities, kidney function, and extent of muscle damage and myoglobinuria. Providers should look for evidence of renal failure, hyperkalemia, metabolic acidosis, and hypocalcemia, and address these issues as needed. As noted above, hypocalcemia is common in crush victims due to sequestration in muscles. However, calcium can be liberated as muscle recovers during the resuscitation phase, and patients may actually be prone to hypercalcemia. Hypercalcemia may complicate further recovery and even promote subsequent cellular destruction, particularly in patients who have received supplemental calcium salts.17,18 Therefore, only symptomatic hypocalcemia should be treated.
Creatine kinase (CK) level is the most sensitive marker for rhabdomyolysis, and it is also a predictor for the development of renal failure. CK is released from muscle cells and begins to rise within 12 hours after injury, peaking at 1-3 days.20 It is slow to degrade, so its levels are more reliable than myoglobin in predicting the severity of muscle injury.16 Oda et al found that 84% of patients with a peak CK greater than 75,000 were very likely to develop acute renal failure and require hemodialysis.7 Oda also reported that the peak CK level increased with the number of limbs injured, so this is can be a simple way to predict severity of injury.
Differential Diagnosis
Crush syndrome should be considered in all patients who present with a history of crushing injury. Usually the patient is able to provide this history and the diagnosis is obvious. However, patients who are comatose or confused may not be able to give a history. Also consider crush syndrome in patients with a history of prolonged immobilization (especially associated with drug overdose or alcohol intoxication).
Management
Much of the literature on management of crush syndrome comes from retrospective studies or case reports of victims of earthquakes and other natural disasters. There are numerous limitations of these studies. Many lack a consistent definition of rhabdomyolysis or acute renal failure, with some lacking a definition at all, which contributes to errors in patient selection and inability to compare patients across studies. Many studies combined different modalities of treatment, making it challenging to isolate the effects of an individual agent. Studies often lacked a control group, which may distort the benefits of a particular regimen. Most studies contained a small number of patients. In 2012, a joint committee consisting of the Renal Disaster Relief Task Force (RDRTF) of the International Society of Nephrology (ISN) and European Renal Best Practice reviewed the literature on crush injuries and added their own expertise to develop a series of guidelines of management.25 These guidelines will be discussed below. See Figure 1 for a proposed outline of early management developed by this committee.
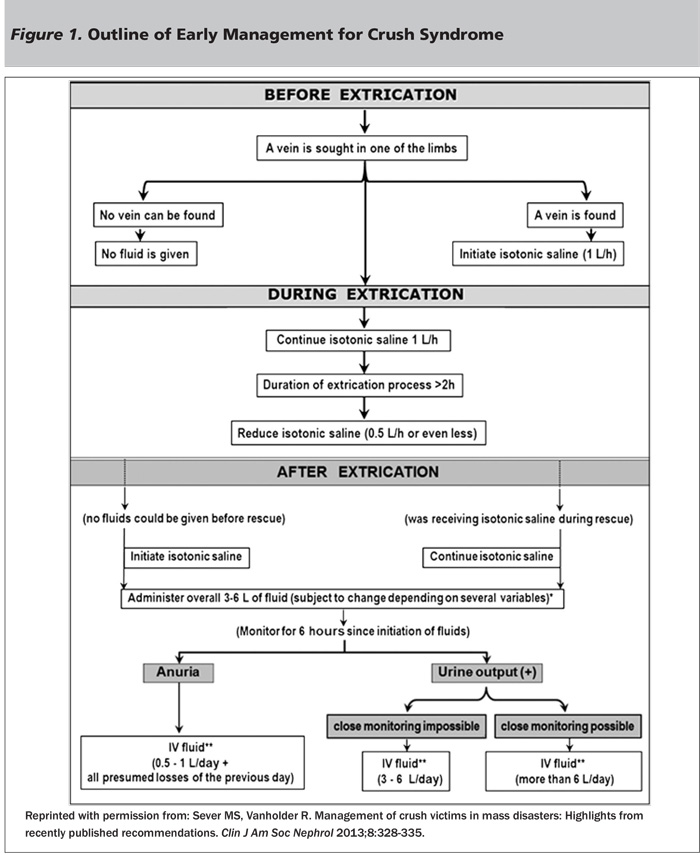
Fluid Resuscitation. The cornerstone of management of crush injuries is fluid resuscitation. Massive fluid resuscitation is required for most patients and should be started pre-extrication, if possible. One of the early protocols for fluid resuscitation was developed based on studies of patients trapped under collapsed buildings in Israel. These retrospective studies were some of the first to demonstrate the importance of immediate fluid resuscitation. In 1979, seven patients with crush injury received fluids at least six hours after extrication (with a mean of 12 hours); despite adequate volume resuscitation post-extrication (an average of 11 liters daily), all patients developed acute renal failure within one day.26 By 1982, a new protocol allowed seven out of eight patients with crush injury to receive fluids during extrication, as well as forced solute-alkaline diuresis within two hours post-extrication. None of the seven patients who received immediate treatment developed acute renal failure, while the one patient who accidentally did not receive immediate treatment did develop acute renal failure within 24 hours.27 Based on these findings, Better and Stein in 1990 recommended an early resuscitation strategy that called for administration of 1.5 liters/hour of isotonic saline during extrication, as soon as an IV could be started.28 Gunal et al reported that after adapting this resuscitation protocol in victims of the 2003 Bingol, Turkey, earthquake, only 25% of patients with crush syndrome required dialysis. The authors postulated that the new resuscitation protocol significantly contributed to the improvement in renal function because a much higher percentage of crush victims required dialysis in the Kobe and Marmara earthquakes — 60.8% and 77%, respectively — in which there was no specific protocol used for resuscitation.5,7 Gunal also noted that the time between rescue and initiation of fluid treatment was significantly longer in the dialyzed victims compared to the nondialyzed ones (9.3 ± 1.7 vs. 3.7 ± 3.3 days).29 Sagheb et al also reported a shorter duration of acute renal failure (7.1 vs 9.4 days) and need for dialysis (1 vs. 6 patients) in crush victims of the Bam earthquake who received early standard resuscitation compared to those who were treated variably.30 The exact amount and type of fluid used in initial resuscitation is still up for debate. The Renal Disaster Relief Task Force (RDRTF) of the International Society of Nephrology (ISN) recommends isotonic saline because it is more readily available in disasters.25 Ringer’s lactate should be avoided because it contains potassium.
Following initial fluid resuscitation, patients should be closely monitored for the next six hours. Many methods may be used for monitoring, depending on the resources available, such as vital signs, clinical appearance, urine output (with a urinary bladder catheter if necessary), signs of external fluid loss (i.e., bleeding, sweating), etc.25 Fluid administration should continue after extrication, with the exact amount given based upon the above factors, as well as time to extrication, age, body mass index, and environmental conditions. The recommended initial daily fluid administration is controversial. Based on retrospective analysis of crush victims of the Bam earthquake, Iraj et al recommended greater than 6 liters for patients with severe rhabdomyolysis and CK levels greater than 15,000, whereas less traumatized victims could receive 3-6 liters.31 Oda et al found that victims of the Marmara earthquake who had CK levels greater than 75,000 had a very high rate of requiring hemodialysis (84.3%). Most of these patients received an average of 3 liters/day of fluid; however, eight of these patients received greater than 6 liters/day of fluid, and only one (11%) developed acute renal failure.7 Better and Stein recommended 12 liters/day of fluid based on their observation that injured muscles can sequester up to 12 liters of fluid in a 48-hour period.28 In Bingol, Turkey, the need for dialysis was prevented in 75% of patients with crush syndrome by administering more than 20 liters/day to each patient.29 Therefore, the clinician must evaluate the clinical situation of the patient, the patient’s age and general health, and resources available to determine the exact amount of the fluid to administer. In general, young healthy adults should be able to tolerate a very high amount of fluid (greater than 12 liters/day), although less (3-6 liters/day) may be appropriate in older or less injured victims or when adequate monitoring is not available.
Patients with persistent hypovolemia, particularly in the case of ongoing blood loss, may require blood transfusion. Analysis of the 1999 Marmara earthquake revealed that 65% of patients with crush syndrome received a blood transfusion.32 A similar percentage (68.8%) of crush victims required blood in the 2003 Bingol earthquake, with an average of 9.2 units per patient.29 Note that blood transfusion can precipitate hyperkalemia, especially in patients with acidosis, hypocalcemia, or hypothermia, and may even lead to cardiac arrest.33
After six hours, patients should be reassessed. If patients remain anuric after adequate fluid resuscitation and the absence of persistent fluid losses, fluid administration should be restricted to 500-1000 milliliters per day, in addition to the estimated fluid losses of the previous day. Crush victims who are not hypovolemic, yet remain anuric, are prone to hypervolemia and may require dialysis if administered excess fluids. Victims of the Marmara earthquake who required dialysis received more fluids than those who did not require dialysis.34 One of the most common indications for dialysis in crush victims is hypervolemia.35 If urine output remains adequate, fluid administration of 3-6 liters/day can continue. Although isotonic fluids are recommended for initial resuscitation, other fluids may be considered post-extrication as discussed below.
Some patients may be delayed in receiving fluid resuscitation due to the chaos that occurs during a natural disaster. These patients may still derive some benefit from aggressive fluid resuscitation post-extrication. Ensari et al studied 27 crush syndrome victims from the Marmara earthquake who had a mean time of 46.5 hours to hospital admission and did not receive prior fluid resuscitation. Upon arrival, these patients received intravenous fluid, mannitol, and diuretics with alkalinization to maintain urine pH greater than 6.5. Only 37% went on to require dialysis, indicating that even late fluid resuscitation is helpful in preventing renal injury.36
Urine Alkalinization. Alkalinization of urine is recommended after extrication to prevent development of renal failure. Alkaluria increases the solubility of myoglobin in urine and prevents its precipitation and trapping within the kidney, and, thus, enhances its excretion.37 Additionally, alkalinization may prevent metabolic acidosis and hyperkalemia. However, these theoretical benefits observed in animal models have not been validated in a randomized, controlled trial of crush victims. Alkalinization can worsen hypocalcemia and deposition of calcium in the tissues. Therefore, arterial pH, calcium levels, serum bicarbonate, and urinary pH should be carefully monitored in patients who receive this treatment.
In 1990, Better et al developed a protocol for management of crush victims, which included alkalinization with sodium bicarbonate. Their protocol recommended the addition of 50 mEq sodium bicarbonate to a hypotonic solution, such as half normal saline, to be administered at 1 liter/hour to maintain a urinary pH greater than 6.5, to begin after initial fluid resuscitation with normal saline.28 This protocol is also recommended by the RDRTF.
Forced Diuresis. Forced diuresis may also decrease myoglobin precipitation through a different mechanism — enhanced renal intratubular flow. This can be achieved with loop diuretics or mannitol. Furosemide was effective in preventing the development of oliguria in 50% of patients with acute renal failure secondary to traumatic rhabdomyolysis from severe beatings in South Africa.38 Loop diuretics, however, are not favored, as they tend to acidify the urine, although the large volume of urine produced may outweigh this disadvantage.15 Mannitol has been discussed more in the literature, but, like urine alkalinization, mannitol administration is largely unproven in the prevention of crush syndrome. In vitro studies have shown that mannitol promotes intravascular volume expansion, dilation of renal vasculature, osmotic diuresis, and possible reduction in intracompartmental pressures.39 Given its potential for side effects, including nephrotoxicity and congestive heart failure, it should be used cautiously.40 Mannitol should not be used in patients with oligoanuria. If mannitol is considered, a test dose should be given first, with repeated doses only if there is a positive response and urine output remains adequate.25 In their 1990 protocol for crush victims, Better et al recommended addition of 20% mannitol at a rate of 1-2g/kg over 4 hours, once urine flow had been established, not to exceed 200 g/day.28
Combination Fluids-Bicarbonate-Mannitol Treatment. A combination of the above treatments (fluid resuscitation, bicarbonate administration, and forced diuresis with mannitol) is recommended by the RDRTF and other protocols, despite a lack of evidence for its efficacy. In 2013, Scharman et al conducted a systematic review of therapy in crush syndrome and found 27 relevant studies, a majority of which were case series or poor-quality cohort studies (73%), with only seven low-quality randomized clinical trials or cohort studies. Of these, 17 studies included only traumatic rhabdomyolysis, four included only non-traumatic rhabdomyolysis, and six included both. Many of the studies claim a benefit from bicarbonate and mannitol, but it is unclear if this was due to the bicarbonate/mannitol, fluids, or if there even was a benefit, as there was no control group.29,38,41,42,43 A few studies did not detect a difference in outcomes from the addition of bicarbonate and mannitol. Homsi et al looked retrospectively at 15 patients with rhabdomyolysis from traumatic and nontraumatic causes who received fluids, bicarbonate, and mannitol versus nine patients who received saline alone. Both groups had a similar baseline creatinine and received the same amount of fluids per hour; none of the patients in either group progressed to develop acute renal failure, and the authors concluded that bicarbonate and mannitol played no role in preventing ARF.44 A retrospective study of 1,771 trauma ICU patients with elevated CK levels did not find a difference in the development of renal failure, need for dialysis, or mortality in patients who received mannitol and bicarbonate versus those who did not.40 In their review of this literature, Scharman et al concluded that evidence was insufficient to recommend mannitol and bicarbonate therapy, and high-quality randomized, controlled trials with a large number of patients need to be performed.45
Hemodialysis. Dialysis can be life-saving in patients with renal failure due to crush syndrome. There are some important logistical considerations. Local dialysis centers may not have the capacity to handle disaster victims. The number of patients requiring dialysis will vary depending on the size of the disaster, but can be overwhelming. In the 1999 Marmara earthquake, 477 patients required dialysis for a total number of 5,137 sessions.46 Dialysis facilities and equipment may be damaged in a disaster, or electricity and/or water supply may not be sufficient to support the equipment. No one mode of dialysis has been shown to be superior in patients with crush syndrome. Intermittent hemodialysis is the most economical approach, as several patients can use one machine per day. Continuous renal replacement therapy may be used in patients with low blood pressure, but utilizes more resources and ideally requires continuous anticoagulation. Peritoneal dialysis is simple and does not require electricity or sterile water; however, it cannot be used in patients with abdominal trauma, is less effective in removing potassium, and patients are more prone to infection due to the less sterile conditions that occur in natural disasters.46 Indications for dialysis include: hyperkalemia resistant to medical therapy, metabolic acidosis resistant to medical therapy, uremia and its complications (i.e., pericarditis), or volume overload.
A retrospective study of victims of the 1995 Kobe earthquake reported that 54% of patients with crush syndrome developed acute renal failure; of these patients, 61% required dialysis.3 Sever et al examined characteristics of crush victims of the 1999 Marmara earthquake and found that 12% of hospitalized patients had some degree of renal insufficiency; of these, 74.6% were treated with dialysis. All types of dialysis modes were used in these patients, although intermittent hemodialysis was by far the most common (94.6%), followed by continuous renal replacement therapy (7.4%), and peritoneal dialysis (1.7%). Some patients required more than one mode. The mean duration of dialysis was 13.4 ± 9.0 days. Interestingly, dialyzed patients actually had a shorter time to extrication than the non-dialyzed. Patients who required dialysis did tend to have more severe illness (sepsis, pneumonia, acute respiratory distress syndrome [ARDS], DIC, need for fasciotomy) than those who were not dialyzed.47
Fasciotomy. Fasciotomy is a mainstay in the treatment of compartment syndrome when compartment pressures rise above 30 mmHg. Until the 1980s, fasciotomies were routinely performed on crush victims with compartment syndrome, often without confirmation of intracompartmental pressures. However, recent conflicting evidence on the efficacy of fasciotomy in crush syndrome has changed this practice. Fasciotomy converts a closed wound to an open wound, so there is a significant risk of infection and sepsis, as well as bleeding.48 In comparison to compartment syndrome from other causes, in crush syndrome, fasciotomy may be less effective because there is already extensive muscle ischemia and necrosis prior to the development of compartment syndrome, and this muscle may not be salvageable.1 Although no randomized trials have been performed, recent observational studies of earthquake victims have shown an association between fasciotomy and increased morbidity. Victims of the Marmara earthquake who underwent fasciotomy compared to those who did not were more likely to develop sepsis (24.8% vs 13%) and require dialysis (83.9% vs 65.2%), although there was no difference in mortality.49 Huang et al found that patients with crush syndrome after the Chi-Chi earthquake who underwent fasciotomy were more likely to develop acute renal failure and require dialysis. Of the 35 patients who underwent fasciotomy, 22.8% developed superficial infection, 45.7% developed deep infection, and 17% went on to require amputation due to an infected fasciotomy site.50
In the 2003 Bingol earthquake, fasciotomies were performed in 68% of crush victims as a routine procedure; 81% were complicated by wound infections.29 Matusoka et al looked at the long-term physical outcome of patients with crush syndrome in the 1995 Kobe earthquake and found no improvement in outcome in patients who underwent fasciotomy. Moreover, 47% who had fasciotomy had severe disability after two years, compared to 16% who did not have the procedure.51 Some studies have reported more favorable outcomes of patients who had fasciotomy performed. Safari et al did not find increased rates of mortality, sepsis, DIC, ARDS, amputation, dialysis sessions, or length of hospital stay in patients who underwent fasciotomy after the Bam earthquake.52 Duman et al also found favorable results in victims of the 1999 Turkey earthquake who underwent fasciotomy, particularly prevention of amputation.53 Nonetheless, in crush victims with acute closed compartment syndrome, fasciotomy should not be routine, and providers should be aware of the risk of infection. Fasciotomy may be indicated when there is significantly elevated intracompartmental pressure greater than 30-40 mmHg (although consider that there may not be devices available for measurement in a disaster situation), need for debridement of necrotic muscle, or loss of peripheral pulses in the absence of a direct arterial injury or systemic hypotension.25 Some studies have shown that osmotic agents, such as mannitol, may be helpful in reducing compartment pressures.54 This approach should be considered if fasciotomy is not performed.48
Amputation. Amputation should be performed only in certain circumstances: the need to free a patient from the disaster site; and an unsalvageable limb or life-threatening sepsis originating from infection of that limb.25 Amputation should not be performed solely to prevent crush syndrome, as it carries a higher mortality. A 2002 study of renal victims of the Marmara earthquake found a mortality of 30.5% in patients who underwent amputation, compared to 12.5% in patients who did not.49 Similarly, tourniquets should not be applied to prevent crush syndrome, but only to control life-threatening bleeding.55
Hyperbaric Oxygen Therapy. Hyperbaric oxygen therapy (HBOt) has been proposed as a relatively new alternative treatment for crush injuries. HBOt may be helpful in treating crush injuries by maintaining tissue oxygenation, increasing tissue perfusion and resorption of edema, blunting the release of free oxygen radicals, and impeding neutrophil adherence to damaged tissues.56 Few clinical studies have been performed on this subject, however. A 2005 systematic literature review analyzed 9 clinical studies that included at least five patients, most of which were retrospective studies or case reports. Eight of nine studies showed a beneficial effect of HBOt, with minimal complications.57 Bouachour et al performed the first randomized, controlled trial of HBOt in patients with crush injuries in 1996. Thirty-six patients with crush injury (mainly from motor vehicle accidents) were randomized to HBOt (a 90-minute protocol at 2.5 atm, twice daily, for six days) versus placebo 24 hours after initial evaluation or surgical procedure. Patients in the HBOt group had an increased rate of complete wound healing (94.4% vs. 55.5%) and decreased rate of new surgical procedures such as skin flaps/grafts or amputation (5.5% vs. 33.3%). When corrected for severity of initial injury, patients older than age 40 with severe crush injury (Gustillo grade III) had the most benefit from HBOt. The authors concluded that HBOt was a useful adjunct to therapy for crush injury, especially in patients with severe injuries.58 Although the evidence is not very robust, HBOt is another treatment modality for crush injuries.
Infection
Infection is a common complication after crush injury. Chen et al analyzed the characteristics of infections in 58 patients with crush syndrome after the Wenchuan earthquake. Infection occurred in 67.2% of patients; wounds (55.2%) and lungs (37.9%) were the most common sites of infection. These infections tended to be serious; 66.7% of those infected developed sepsis. In wound infections, Acinetobacter baumannii and Pseudomonas aeruginosa were the most common bacteria isolated. Patients tended to develop infection early in their hospital stay — 66.7% of infections occurred within 48 hours of admission. Risk factors for infection included duration under the rubble, duration of renal impairment, and fasciotomy.59
Therefore, crush injury patients require a thorough physical evaluation. Wounds, if present, should be considered contaminated and subsequently washed with soap and water and/or debrided as soon as possible. Patients should receive tetanus prophylaxis. Antibiotics should not be administered empirically unless wounds appear infected or surgical procedures are planned. Antibiotics should be targeted toward gram-positive skin flora (staphylococci and streptococci species) and/or anaerobes. A first-generation cephalosporin such as cefazolin is generally effective for most cases. For patients with open fractures that require gram-negative coverage, ciprofloxacin is a good choice. Aminoglycosides are often avoided due to their toxic effects on the kidney. Antibiotics should be renally dosed when appropriate.25
Disposition
Consider all disaster victims, and all patients who have sustained an appropriate mechanism, at risk for crush syndrome, even those with no apparent injury. Oda et al noted that some patients affected by the Kobe earthquake in 1995 appeared stable at the scene and were sent to shelters, then presented to a hospital several days later with renal failure. The risk for development of crush syndrome may not always be apparent; in fact, a majority of patients (69%) with crush syndrome did not have any fractures, and 11.6% of these patients died.7 Therefore, most patients with crush injury should be admitted to the hospital. However, this may not be feasible in a mass casualty situation. If patients must be discharged due to lack of hospital beds, they should be instructed to check the volume and color of their urine for at least three days and return for any signs of renal failure (nausea/vomiting, shortness of breath, edema). Crush victims should be treated in a facility with access to surgeons/operating rooms, dialysis machines, and an intensive care unit. Local facilities near a disaster area may be damaged or suffer from loss of personnel, so clinicians should be prepared to transfer patients for logistical reasons.
Summary
Crush injury may occur after natural disasters such as earthquakes, terrorist acts, building collapse, and motor vehicle accidents. Carefully assess patients with drug overdose or alcohol intoxication for signs of crush injury. Crush syndrome is a systemic response to local muscle damage characterized by rhabdomyolysis, acute renal failure, hypotension, hyperkalemia, metabolic acidosis, and hypocalcemia. Patients are also at risk for developing compartment syndrome. CK is the most sensitive test for detecting rhabdomyolysis and may be used to predict risk for developing acute renal failure. Patients should be administered fluids during extrication to prevent development of renal failure. Urine alkalinization and forced diuresis with mannitol should be considered to prevent myoglobin precipitation in the renal tubules. Fasciotomy should not be performed routinely due to the risk of infection. Clinicians and facilities should develop protocols for natural disaster planning, as crush victims often require many resources that are prone to disruption. With early fluid administration, anticipation of complications of crush syndrome, and resource planning, many deaths can be prevented.
References
- 1.Reis ND, Better OS. Mechanical muscle-crush injury and acute muscle-crush compartment syndrome. J Bone Joint Surg 2005;87-B:450-453.
- 2.Bywaters EGL, Beall D. Crush injuries with impairment of renal function. Br Med J 1941;1(4185):427-432.
- 3.Tanaka H, Oda J, Iwai A. Morbidity and mortality of hospitalized patients after the 1995 Hanshin-Awaji earthquake. Am J Emerg Med 1999;17(2):186-191.
- 4.Bartal C, Zeller L, Miskin I, et al. Crush syndrome: Saving more lives in disasters: Lessons learned from the early-response phase in Haiti. Arch Intern Med 2011;171(7):694-696.
- 5.Sever MS, Erek E, Vanholder R, et al. The Marmara earthquake: Epidemiological analysis of the victims with nephrological problems. Kidney Int 2001;60(3):1114-1123.
- 6.Ukai T. The great Hanshin-Awaji earthquake and the problems with emergency medical care. Ren Fail 1997;19:633-645.
-
7.Oda J, Tanaka H, Yoshioka T, et al. Analysis of 372 patients with crush syndrome caused by the Hanshin-Awaji earthquake. J Trauma 1997;42(3):
470-476. - 8.Smith J, Greaves I. Crush injury and crush syndrome: A review. J Trauma 2003;54(5 Suppl):S226-S230.
- 9.Sever MS, Erek E, Vanholder R, et al. Lessons learned from the Marmara disaster: Time period under the rubble. Crit Care Med 2002;30(11):2443-2449.
- 10.Hatamizadeh P, Najafi I, Vanholder R, et al. Epidemiologic aspects of the Bam earthquake in Iran: The nephrologic perspective. Am J Kidney Dis 2006;47(3):428-438.
- 11.Malinoski DJ, Slater MS, Mullins RJ. Crush injury and rhabdomyolysis. Crit Care Clin 2004;20(1):171-192.
- 12.Brumback RA, Feeback DL, Leech RW. Rhabdomyolysis in childhood: A primer on normal muscle function and selected metabolic myopathies characterized by disordered energy production. Pediatr Clin North Am 1992;39:821-858.
- 13.Zager RA. Rhabdomyolysis and myohemoglobinuric acute renal failure. Kidney Int 1996;49:314-326.
- 14.Ashkenazi I, Isakovich B, Kluger Y, et al. Prehospital management of earthquake casualties buried under rubble. Prehosp Disaster Med 2005;20(2):122-133.
- 15.Gonzalez D. Crush syndrome. Crit Care Med 2005;33(S1):S34-S41.
- 16.Vanholder R, Sever MS, Erek E. Rhabdomyolysis. J Am Soc Nephrol 2000;11(8):1553-1561.
- 17.Knochel JP. Serum calcium derangements in rhabdomyolysis. N Engl J Med 1981;305(3):161-163.
- 18.Lane JT, Boudreau RJ, Kinlaw WB. Disappearance of muscular calcium deposits during resolution of prolonged rhabdomyolysis-induced hypercalcemia. Am J Med 1990;89(4):523-525.
- 19.Owen CA, Mubarak SJ, Hargens AR, et al. Intramuscular pressures with limb compression: Clarification of the pathogenesis of the drug-induced muscle-compartment syndrome. N Engl J Med 1979;300:1169-1172.
- 20.Huerta-Alardin AL, Varon J, Marik PE. Bench-to-bedside review: Rhabdomyolysis — an overview for clinicians. Crit Care 2005;9(2):156-169.
- 21.Aoki N, Demsar J, Zupan B, et al. Predictive model for estimating risk of crush syndrome: A data mining approach. J Trauma 2007;62(4):940-945.
- 22.He Q, Wang F, Li G, et al. Crush syndrome and acute kidney injury in the Wenchuan earthquake. J Trauma 2011;70(5):1213-1217.
-
23.Vanholder R, Borniche D, Claus S, et al. When the earth trembles in the Americas: The experience of Haiti and Chile 2010. Nephron Clin Pract 2011;117(3):
c184-197 - 24.Sever MS, Erek E, Vanholder R, et al. Serum potassium in the crush syndrome victims of the Marmara disaster. Clin Nephrol 2003;59(5):326-333.
- 25.Sever MS, Vanholder R, RDRTF of ISN Work Group on Recommendations for the Management of Crush Victims in Mass Disasters. Recommendation for the management of crush victims in mass disasters. Nephrol Dial Transplant 2012;27 Suppl 1:i1-67.
- 26.Reis ND, Michaelson M. Crush injury to the lower limbs: Treatment of the local injury. J Bone Joint Surg [Am] 1986;68:414-418.
- 27.Ron D, Taitelman U, Michaelson M, et al. Prevention of acute renal failure in traumatic rhabdomyolysis. Arch Intern Med 1984;144:277-280.
- 28.Better OS, Stein JH. Early management of shock and prophylaxis of acute renal failure in traumatic rhabdomyolysis. N Engl J Med 1990;322:825-829.
- 29.Gunal AI, Celiker H, Dogukan A, et al. Early and vigorous fluid resuscitation prevents acute renal failure in crush victims of catastrophic earthquakes. J Am Soc Nephrol 2004;15:1862-1867.
-
30.Sagheb MM, Sharifian M, Roozbeh J, et al. Effect of fluid therapy on prevention of acute renal failure in Bam earthquake crush victims. Ren Fail 2008;30(9):
831-835. - 31.Iraj N, Saeed S, Mostafa H. Prophylactic fluid therapy in crushed victims of Bam earthquake. Am J Emerg Med 2011;29(7):738-742.
- 32.Kazancioglu R, Pinarbasi B, Esen BA et al. The need for blood products in patients with crush syndrome. Am J Disaster Med 2010;5(5):295-301.
- 33.Smith HM, Farrow SJ, Ackerman JD. Cardiac arrests associated with hyperkalemia during red blood cell transfusion: A case series. Anesth Analg 2008;106(4):1062-1069.
- 34.Sever MS, Erek E, Vanholder R, et al. Treatment modalities and outcomes of renal victims of the Marmara earthquake. Nephron 2002;92(1):64-71.
- 35.Sever MS. The crush syndrome (and lessons learned from the Marmara earthquake). S. Karger AG: Basel, 2005
- 36.Ensari C, Tufekcioglu O, Ayli D et al. Response to delayed fluid therapy in crush syndrome. Nephron 2002;92(4):941-943.
- 37.Zager RA. Studies of mechanisms and protective maneuvers in myoglobinuric acute renal injury. Lab Invest 1989;60(5):619-629.
- 38.Knottenbelt JD. Traumatic rhabdomyolysis from severe beating — experience of volume diuresis in 200 patients. J Trauma 1994;37(2):214-219.
-
39.Moore KP, Holt S, Patel RP, et al. A causative role for redox cycling of myoglobin and its inhibition by alkalinization in the pathogenesis and treatment of rhabdomyolysis-induced renal failure.
J Biol Chem 1998;273:31731-7. - 40.Brown CV, Rhee P, Chan L, et al. Preventing renal failure in patients with rhabdomyolysis: Do bicarbonate and mannitol make a difference? J Trauma 2004;56(6):1191-1196.
- 41.Altintepe L, Guney I, Tonbul Z. Early and intensive fluid replacement prevents acute renal failure in the crush cases associated with spontaneous collapse of an apartment in Konya. Ren Fail 2007;29(6):737-741.
- 42.Eneas JF, Schoenfeld PY, Humphreys MH. The effect of infusion of mannitol-sodium bicarbonate on the clinical course of myoglobinuria. Arch Intern Med 1979;139(7):801-805.
- 43.Michaelson M, Taitelman U, Bshouty Z, et al. Crush syndrome: Experience from the Lebanon War, 1982. Isr J Med Sci 1984;20(4):305-307.
- 44. Homsi E, Barreiro MF, Orlando JM, et al. Prophylaxis of acute renal failure in patients with rhabdomyolysis. Ren Fail 1997;19(2):283-288.
- 45.Scharman EJ, Troutman WG. Prevention of kidney injury following rhabdomyolysis: A systematic review. Ann Pharmacother 2013;47(1):90-105.
- 46.Sever MS, Vanholder R, Lameire N. Management of crush-related injuries after disasters. N Engl J Med 2006;354:1052-1063.
-
47.Sever MS, Erek E, Vanholder R, et al. Renal replacement therapies in the aftermath of the catastrophic Marmara earthquake. Kidney Int 2002;62(6):
2264-2271. - 48.Better OS, Rubinstein I, Reis DN. Muscle crush compartment syndrome: Fulminant local edema with threatening systemic effects. Kidney Int 2003;63:1155-1157.
- 49.Sever MS, Erek E, Vanholder R, et al. Clinical findings in the renal victims of a catastrophic disaster: The Marmara earthquake. Nephrol Dial Transplant 2002;17(11):1942-1949.
- 50.Huang KC, Lee TS, Lin YM, et al. Clinical features and outcome of crush syndrome caused by the Chi-Chi earthquake. J Formos Med Assoc 2002;101(4):249-256.
-
51.Matusoka T, Yoshioka T, Tanaka H, et al. Long-term physical outcome of patients who suffered crush syndrome after the 1995 Hanshin-Awaji earthquake: Prognostic indicators in retrospect.
J Trauma 2002;52(1):33-39. - 52.Safari S, Najafi I, Hosseini M. Outcomes of fasciotomy in patients with crush-induced acute kidney injury after Bam earthquake. Iran J Kidney Dis 2011;5(1):25-28.
-
53.Duman H, Kulahci Y, Sengezer M. Fasciotomy in crush injury resulting from prolonged pressure in an earthquake in Turkey. Emerg Med J 2003;20(3):
251-252. - 54.Daniels M, Reichman J, Brezis M. Mannitol treatment for acute compartment syndrome. Nephron 1998;79(4):492-493.
- 55.Jagodzinski NA, Weerasinghe C, Porter K. Crush injuries and crush syndrome — a review. Part 2: The local injury. Trauma 2010;12:133–148.
- 56.Buettner MF, Wolkenhauer D. Hyperbaric oxygen therapy in the treatment of open fractures and crush injuries. Emerg Med Clin North Am 2007;25(1):177-188.
- 57.Garcia-Covarrubias L, McSwain NE, Van Meter K, et al. Adjuvant hyperbaric oxygen therapy in the management of crush injury and traumatic ischemia: An evidence-based approach. Am Surg 2005;71(2):144-151.
-
58.Bouachour G, Cronier P, Gouello JP, et al. Hyperbaric oxygen therapy in the management of crush injuries: A randomized, double-blind placebo-controlled clinical trial. J Trauma 1996;41(2):
333-339. - 59.Chen X, Zhong H, Fu P, et al. Infections in crush syndrome: A retrospective observational study after the Wenchuan earthquake. Emerg Med J 2011;28(1):14-17.
MONOGRAPH: In the U.S., alcohol intoxication associated with prolonged muscle compression and/or seizures is the most common cause of traumatic rhabdomyolysis.
Subscribe Now for Access
You have reached your article limit for the month. We hope you found our articles both enjoyable and insightful. For information on new subscriptions, product trials, alternative billing arrangements or group and site discounts please call 800-688-2421. We look forward to having you as a long-term member of the Relias Media community.
