Maximizing Survival from Severe Traumatic Brain Injury: Applying Guidelines to Clinical Practice: Part I
Maximizing Survival from Severe Traumatic Brain Injury: Applying Guidelines to Clinical Practice: Part I
Head injury can mean many things, from a bump on the forehead to an open wound with extruding brain. This review focuses on patients with severe closed head injuries. Like many other areas of medicine, we have good evidence for what is the best care. The principles may seem simple, but applying them is not always easy. The challenge is to deliver optimal care consistently and continuously, from the scene, during transport, in the emergency department, through the operating room and ICU. This is the first of a two-part series on severe traumatic brain injury, focusing on the evidence for optimal care.
J. Stephan Stapczynski, MD, Editor
Introduction
Case Scenario. Paramedics are called to a private residence in response to a 54-year-old male. His wife reports that he fell 5 feet from a step ladder and struck the back of his head. The patient is a marathon runner who recently has been diagnosed with atrial fibrillation for which he is taking warfarin. Upon emergency medical service (EMS) arrival, he has sonorous respirations, symmetric pupils, and a Glasgow Coma Score (GCS) of 7: E1 M4 V2. His systolic blood pressure (SBP) is 85 mmHg, and his heart rate is 140 beats/minute and irregular. His oxygen saturation is 88%. Paramedics identify his low GCS and consider endotracheal intubation (ETI), but, given his clenched jaw and their short transport time, they elect to treat him with a non-rebreather facemask and high-flow oxygen. The patient is placed in a rigid cervical collar for immobilization and then transferred onto a backboard while maintaining cervical spine precautions. His oxygen saturations climb to the mid 90s. Five hundred milliliters of normal saline is administered, and his SBP rises to 105 mmHg with a drop in his heart rate to the 120s. He is transported to the nearest Level I trauma center with neurosurgical capabilities.
Upon arrival, the trauma team observes that the patient's pupils are symmetric and reactive. During preoxygenation, stat labs are sent, and an electrocardiogram demonstrates atrial fibrillation. The emergency physician successfully performs rapid sequence intubation (RSI) after premedication with lidocaine. The patient is taken emergently to radiology on the monitor with an advanced trauma life support (ATLS) trained nurse for non-contrast CT scans of the head and cervical spine. The neurosurgical consultant is simultaneously called. Upon return from CT, the patient is noted to have a dilated, non-reactive right pupil and begins to posture. The ventilator is set for a target PCO2 of 30-35 using continuous capnography monitoring for guidance. The CT scan reveals a large right-sided subdural hematoma with midline shift and evidence of herniation. The patient's INR returns at 3.0. The neurosurgeon is now at the bedside. The patient's SBP is 110 mmHg, and his heart rate is 100 beats/minute. The neurosurgeon requests mannitol, noting that his cardiovascular status appears to have stabilized. Four units of fresh frozen plasma (FFP) are ordered, and the patient is transported to the operating room for evacuation of his subdural hematoma.
Epidemiology and Societal Impact of Traumatic Brain Injury
Traumatic brain injury (TBI) is a leading cause of death and disability worldwide. In the United States, 1.4 million cases of TBI are reported annually, with 1.1 million cases treated in the emergency department and 235,000 cases hospitalized each year.1,2 The incidence of TBI peaks at ages 15-24 years, with smaller peaks at the extremes of age, younger than 5 and older than 70 years of age.3 TBIs frequently are the result of falls and motor vehicle collisions, which comprise 28% and 20%, respectively.1 Fall-associated TBI rates are highest for children ages 0-4 years and adults 75 years and older, while motor vehicle-related TBI rates are highest in adolescents aged 15-19.1 Child abuse is the leading cause of death from TBI in those younger than 2 years of age.4 TBI is prevalent on the battlefield, with more than 60% of military personnel returning to the Walter Reed Army Medical Center from Operation Enduring Freedom/Operation Iraqi Freedom having suffered a blast-induced TBI.5
Each year in the United States, approximately 50,000 people die from TBI, 2,685 of whom are younger than 15 years old.1 Nearly half of those who die from TBI do so in the first two hours.6 In children and adults, TBI is the most important determinant of outcome from trauma.6 The mortality rate from blunt trauma without TBI is 1%, and with TBI it skyrockets to 30%.7
Severe Traumatic Brain Injury. TBI is most often classified by using the GCS.8 The GCS is a sum score of three aspects of neurologic function: eye opening, verbal response, and motor response. The scores for each category should be reported separately and are subsequently added together, with the lowest possible total being three and the highest 15. It should be noted that the GCS has several limitations, such as the level of consciousness being impacted by paralysis, intoxication, and medical sedation.9,10 A GCS of 8 or less is considered severe TBI, GCS 9-13 moderate TBI, and GCS 14-15 mild TBI.11,12 Mild TBI comprises 80% of TBI cases, while moderate and severe TBI each contribute 10%.3
Primary and Secondary Brain Injury. TBI is described by both primary and secondary brain injury. Primary brain injury refers to the damage that occurs or is initiated at the time of the traumatic event, while secondary brain injury is a result of factors that occur after the initial trauma and either worsen injury or negatively influence outcome. Primary brain injury is a result of external forces to the head (direct impact, rapid acceleration and deceleration, penetrating object, or blast waves) that cause injury to neurologic and vascular tissue both locally and remote from the site of injury. Many of the deleterious mechanisms leading to secondary injury, such as disruption of cellular homeostasis, excitotoxicity of glutamate and aspartate, and superoxide generation, are not currently amenable to therapy.6 As such, clinical efforts are aimed at minimizing second insults to the brain, including hypotension, hypoxia, hypocapnea, pyrexia, coagulopathy, and anemia. Data from the Traumatic Coma Data Bank (TCDB) demonstrate that a single hypotensive measurement (SBP < 90 mmHg) occurred in one-third of severe TBI victims between injury and resuscitation and was independently associated with a doubling of mortality.13 Similar findings have been shown in the pediatric population.14 Hypoxia (O2 saturation < 90%) in both the prehospital and in-hospital settings is also associated with poor outcomes.13,15 While induced hypocapnea (PaCO2 < 35 mmHg) is used as a salvage technique during herniation, the potential benefits of prophylactic hypocapnea to decrease intracranial pressure (ICP) are more than offset by reductions in cerebral blood flow (CBF) and subsequent ischemia.16-20 Pyrexia (T > 100.4 °F) is common and is also strongly correlated with poor outcomes.15 (See Table 1.)
 |
Anatomy and Pathophysiology
The brain is semisolid and weighs approximately 3 lbs. Despite its diminutive size, it receives about 15% of total cardiac output and accounts for approximately 20% of total body oxygen consumption.12,21
A normally functioning brain maintains its blood flow through autoregulation. The cerebral perfusion pressure (CPP) serves as an estimate of CBF and is defined as the difference between the mean arterial pressure (MAP) and the ICP (CPP=MAP-ICP). The brain with a MAP between 60-150 is able to regulate cerebral blood flow by constricting and dilating the vasculature depending on the conditions.22 Hypertension, alkalemia, and hypocapnea result in cerebral vasoconstriction and decreased CBF, while hypotension, acidemia, and hypercapnea result in vasodilation and increased CBF. The volume of the skull is fixed and, as such, the Monro-Kellie Doctrine states that the ICP is directly related to the volume of the intracranial contents. The normal intracranial contents are brain parenchyma, blood, and cerebrospinal fluid (CSF).6 Cerebral edema after TBI can increase brain volume and is either vasogenic or cytotoxic. Vasogenic edema is the result of a compromised blood-brain barrier, and cytotoxic edema, the primary culprit in TBI, is a result of brain cell death.6,12,23 Blood volume can increase for several reasons, including venous outflow obstruction, metabolic derangements (e.g. pyrexia and seizures), or vasodilatation from loss of autoregulation.6 Finally, CSF volume can increase if drainage is obstructed, such as with an intraventricular lesion or obstruction of outflow through the posterior fossa.6
Intracranial hypertension from any of the aforementioned causes can result in both ischemia and herniation. Ischemia is caused by a decreased CPP and ultimately leads to cell death. Herniation occurs when there is a pressure gradient across a fixed anatomic barrier. This most commonly occurs at the tentorial membrane separating the cerebral hemispheres from the posterior fossa. When transtentorial herniation (often referred to as uncal herniation) occurs, pressure is exerted on the brainstem, the cranial nerves, and the local vasculature. This may result in anisocoria, posturing, autonomic disturbances, and/or death.6 (See Figure 1.)
 |
Brain Trauma Foundation Guidelines. During the past 30 years, it has been demonstrated that compliance with evidence-based in-hospital protocols that emphasize monitoring and the maintenance of cerebral perfusion has resulted in a drop in TBI mortality from 50% to less than 25%.24-30 In 2007, the Brain Trauma Foundation (BTF) published the third edition of the Guidelines for the Management of Severe Traumatic Brain Injury.31 The BTF is a national non-profit organization of neurosurgical experts that was founded to improve the outcomes of patients with TBI by creating evidence-based best practice guidelines, performing clinical research, and educating health professionals.32 Since the publication of the initial BTF guidelines, there have been several studies demonstrating that BTF guideline-based care decreases mortality, length of hospital stay, and costs while improving functional outcomes.24,25,29 A recent study showed that the use of BTF guidelines for treatment of severe TBI improved health outcomes and costs and suggested that widespread implementation of these guidelines would result in substantial savings in medical costs, annual rehabilitation costs, and lifetime societal costs.33 Despite widespread dissemination of the in-hospital guidelines, adoption has been limited.27 In 2007, the BTF published the first separate Guidelines for Prehospital Management of Traumatic Brain Injury (evidence through July 2006) in Prehospital Emergency Care.34 Little is known about the utilization of guideline-based care in the prehospital setting.
Prehospital Care
Brain Trauma Foundation. There is growing evidence that what happens in the initial "platinum 10 minutes" following TBI may profoundly impact the effectiveness of subsequent definitive care. EMS providers typically are the first healthcare providers to treat patients with TBI, making their assessment and treatment a critical link in the chain of survival.35 In accordance with the Guidelines for the Prehospital Management of Traumatic Brain Injury, the following section on the prehospital management of severe TBI will be divided into "assessment" and "treatment" topics.34
Assessment. Maintaining adequate oxygenation and blood pressure are critical steps in maximizing outcomes in patients with TBI.36 Given the well-documented negative impact of hypotension and hypoxemia following TBI, patients with suspected TBI should be monitored closely in the prehospital setting and have measures taken to prevent both conditions.13,14 Guidelines state that oxygen saturation levels and blood pressure (systolic and diastolic) should be monitored as often as possible, preferably continuously. For children, appropriate size pediatric oximeters and blood pressure cuffs are recommended. Hypoxemia is defined as arterial hemoglobin oxygen saturation < 90% in both children and adults. Adult hypotension has been defined as SBP below 90 mmHg, while for children it is age-dependent, ranging from < 60 mmHg for those between 0-28 days old to < 90 for those aged 10 years or older.34
The GCS should be evaluated repeatedly to monitor improvement or deterioration of the patient's condition (pediatric GCS for children < 2 years of age). (See Table 2.) It should be measured after the ABCs are assessed and treated and prior to administering sedatives or paralytic agents (or after metabolism of these drugs). Pupils should be assessed in both adults and children to aid in the subsequent determination of diagnosis, treatment, and prognosis. They should be measured after the patient has been resuscitated and stabilized. Asymmetry is defined as > 1 mm difference in diameter. A fixed pupil is defined as < 1 mm response to bright light.34
 |
Standard initial assessments of comatose patients also should be performed, such as a blood glucose measurement.
Treatment. The primary goals in the prehospital management of TBI are to prevent hypotension and hypoxia, to avoid hyper- and hypocapnia, and to triage to the appropriate center.34,37 In the prehospital setting, hypoxia is discovered in 44-55% of cases, and hypotension is found in 20-30%.37-39 The introduction of a prehospital system capable of normalizing blood pressure and oxygenation has been associated with improved outcomes in various locations.40,41
Hypoxemia (oxygen saturation < 90%) should be corrected immediately upon detection. A definitive airway should be established in patients who have a GCS of 8 or less and either the inability to maintain an adequate airway or hypoxemia not corrected with supplemental oxygen. The BTF guidelines state that ground-transported patients in an urban environment who are spontaneously breathing and are maintaining their oxygen saturations above 90% on supplemental oxygen should not be treated with paralytic medications to assist ETI.34 When RSI is indicated, protocols should include monitoring serial blood pressure, oxygenation, and end-tidal CO2 (ETCO2). Endotracheal tube placement should be confirmed via lung auscultation and ETCO2 determination. Normal breathing rates confirmed by ETCO2 of 35-40 mmHg should be maintained and hyperventilation (ETCO2 < 35 mmHg) should be absolutely avoided unless the patient shows signs of cerebral herniation.34
Hypotensive patients with TBI should be treated with isotonic fluids. The BTF guidelines support hypertonic fluid resuscitation as a treatment option for adult TBI patients with a GCS < 8.34 It should be noted, however, that a recent large Resuscitation Outcomes Consortium (ROC) trial of prehospital hypertonic saline (HTS) for severe TBI victims was halted by The National Heart, Lung and Blood Institute (NHLBI) because HTS did not improve outcomes when compared to standard treatment with normal saline.42
Patients should be assessed frequently for signs of acute herniation, such as dilated, unreactive, or asymmetric pupils; a motor examination with extensor posturing or no response; and acute neurological deterioration. While prophylactic hyperventilation should be avoided, hyperventilation therapy might be necessary for brief periods in patients who are normoventilated, well oxygenated, and normotensive and still have signs of herniation. Hyperventilation should be titrated to effect and be discontinued when clinical signs of herniation resolve. The goal of hyperventilation is an ETCO2 of 30-35 mmHg. The current literature does not support the use of mannitol or HTS to treat herniation in the prehospital setting.34
To ensure the best possible outcomes for TBI patients, the BTF recommends that all regions should have an organized trauma care system and protocols in place to direct EMS personnel regarding destination decisions for patients with severe TBI. Patients with TBI should be transported directly to a facility with immediate CT availability, neurosurgical capability, and the ability to monitor ICP and treat intracranial hypertension. The mode of transport should minimize prehospital time. Pediatric patients should be transported directly to a pediatric trauma center when available. If a pediatric trauma center is not available, preference should be given to an adult trauma center with added qualifications to treat children.34
Patel and colleagues demonstrated a doubling of the odds of death for patients with severe TBI treated in non-neurosurgical centers versus neurosurgical centers.43 Maas et al. suggest that all treatable patients with severe TBI be treated in large neurotrauma centers that offer surgical therapy and access to neurocritical care.44-47 Greater experience treating severe TBI leads to higher quality care and better outcomes.48-50
Prehospital RSI. Several recent studies have suggested that prehospital RSI is associated with worse outcomes.51-55 Subsequent analysis has shown that RSI per se was not directly responsible, but that hypoxia during induction and inadvertent hyperventilation after intubation were common and likely contributed to the poorer outcomes in the RSI groups.56-59 In fact, prehospital RSI can improve outcomes in severe TBI.60-64
Hyperventilation/hypocapnea results in vasoconstriction of the cerebral arterioles, thereby reducing CBF, cerebral blood volume (CBV), ICP, and cerebral oxygen delivery. In reality, the potentially favorable drop in ICP due to hypocapnea is more than offset by the negative consequences of decreased CBF with subsequent cerebral ischemia and secondary injury of the traumatized brain.16-20 Positive pressure ventilation may cause additional secondary insults by increasing intrathoracic pressure, which decreases venous return to the heart. This can result in systemic hypotension and increased ICP by causing congestion in the jugular venous system.16,65,66 Additionally, an increase in intrathoracic pressure is transmitted through the CSF and the spinal veins, causing an increase in ICP.67
As early as 1991, randomized controlled trial evidence of negative outcomes from prolonged hyperventila- tion in severe TBI patients led to the recommendation that routine hyperventilation be avoided.31,34,68 Despite the evidence and the BTF recommendation, hyperventilation is common during the prehospital resuscitation of TBI patients.56,62,69 In addition to hyperventilation, hypoventilation also has deleterious effect on the injured brain. A PaCO2 of > 45 mmHg (severe hypercapnea) on arrival at the ED is associated with hypotension, hypoxia, metabolic acidosis, and a significant increase in mortality.55 Prehospital airway management must concentrate not only on the quality of the intubation, but on the subsequent ventilation management.61 Responders must be trained to ventilate at a set rate and tidal volume. More controlled ventilation methods such as autoventilators should be considered to ensure maintenance of target ETCO2 without inadvertent hyper- or hypoventilation.16 The target ETCO2 for prehospital patients with TBI is 35-40 mmHg.34,70 Patients arriving at the hospital within the target range have better outcomes.31,55
The BTF guidelines recommend prehospital end-tidal capnography as a way to target ventilation in cases of severe TBI.34 It must be noted that while ETCO2 values match PaCO2 values closely in the operating room, there is question about how well they correlate in the emergency setting.71-73 Correlation appears to be best in patients with isolated TBI.16 Further research is necessary to establish firm ventilation recommendations based on this monitoring method.16
The BTF assembled a panel of experts to interpret the existing literature and to make recommendations regarding the use of RSI in the field for severe TBI. Table 3 outlines their recommendations in question-and-answer format.61
 |
Emergency Department Care
Initial Evaluation. The cornerstone of the initial emergency department management of severe TBI remains the ABCs and the maintenance of spinal immobilization. Estimates suggest that 35-60% of severe TBI victims have another traumatic injury.74,75 If the EMS report is suggestive of severe TBI, the trauma team should be activated when available. Upon arrival, the lead physician should perform the primary survey with continuous maintenance of cervical spine precautions. Neurologic status should be assessed early to establish a baseline. Attention should be focused on the GCS and pupillary exams (prior to RSI and administration of paralytics). Signs of herniation, such as pupillary asymmetry and loss of reaction to light, should be identified rapidly. In a patient not responsive to verbal cues, a noxious stimulus should be applied to all four limbs. Interventions such as ETI should be performed as indicated during the standard ATLS sequence. A report should be taken from EMS providers to ascertain any information that might help in management. Vital signs, ETCO2, and blood glucose should be obtained. A thorough secondary survey should be performed as soon as the initial survey is complete to assess for other injuries.76
History. The initial history must be detailed and accurate but also efficient. An effective method is the AMPLE categories. Allergies, Medications, Past medical history, Last meal and Events/Environment related to the injury.76 A history from EMS personnel, family, and bystanders should be obtained when available. Important additional questions include those related to the mechanism of injury, loss of consciousness, history of seizures, and alcohol or drug intoxication.
Physical Examination. A complete trauma examination should be performed for severe TBI patients, with special attention paid to coma scoring and the pupil exam. The GCS was developed by neurosurgeons Teasdale and Jennett at Glasgow University in 1974 to gauge and communicate coma progression and to predict outcome.8,77-80 While the GCS remains the most commonly used coma score and is advocated by the BTF, it should be noted that it is not without limitations. For example, if a patient undergoes field RSI, the chemical sedation and paralysis render the scale of little utility.81 In its original form, the initial score was obtained at 6 hours after injury, giving the injury time to declare itself.8 As such, early GCS scores can be inaccurate and can be altered significantly by alcohol and drug ingestion. Winkler et al. and Bazarian et al. both have shown that the prehospital GCS can differ significantly from the GCS upon arrival at the emergency department.82,83
There are other concerns with the GCS score. Gill et al. showed that the inter-rater reliability is low, and Teoh et al. have demonstrated that GCS scores with different permutations of eye, verbal, and motor can have different meanings.84,85 Finally, and perhaps most importantly, using the GCS alone to make treatment decisions can lead to unnecessary and perhaps deleterious intubations. In the San Diego Paramedic RSI trial, Davis et al. showed that when using GCS alone to determine the need for intubation, 27% of patients who were intubated did not have a significant head injury.51,86 This was particularly common in the intoxicated cohort. Additionally, many of their historical controls who would have met GCS < 8 intubation criteria were oxygenating and ventilating adequately on hospital arrival.51,86
There are a number of coma scoring systems that attempt to balance simplicity and ease of use with adequate detail to provide utility. The FOUR (Full Outline of UnResponsiveness) Score is a new coma score that shows promise.87 (See Table 2.) It has four components: eye response, motor response, brainstem reflexes, and respirations. Each component has a score between 0-4, and the components are not totaled. The FOUR Score coma scale has been validated in the neurointensive care unit and in the emergency department by both physicians and nurses and appears to be performed as reliably as the GCS while providing more detailed information.87,88
Differential Diagnosis. As in all cases of critical illness, it is imperative that a broad differential diagnosis is maintained. The possibility of medical illness as the precipitant of the trauma always should be considered, (e.g., syncope, cardiac arrest, seizure, hypoglycemia, stroke). It has been estimated that more than 60% of TBIs are related to the abuse of alcohol or another substance.89 Hypoglycemia can lead to traumatic injury as a result of altered mental status and, if left undetected, can lead to permanent neurologic damage and death.90
There are a number of different types of TBI, and their management can vary. Early neuroimaging is used to distinguish between epidural hematoma, diffuse axonal injury (DAI), intraparenchymal hemorrhage, subarachnoid hemorrhage, and subdural hematoma.
Laboratory Tests. Many severely head-injured patients have suffered another traumatic injury.74,75 The laboratory studies obtained in severe TBI are those that are routinely obtained for major trauma victims. Traditional laboratory tests include the complete blood count, metabolic panel, coagulation panel, arterial blood gas, serum lactate level, and the blood type. An evaluation of serum alcohol and a urine drug screen also can be beneficial to exclude alternative etiologies of altered mental status.
Imaging. The imaging modality of choice is the non-contrast CT scan of the head.91 CT scans are widely and rapidly available and are highly sensitive in the detection of acute hemorrhage, mass effect, midline shift, and bony injuries.92 The CT scan should be obtained as early as possible with the intent of detecting lesions that require immediate neurosurgical intervention. Monitoring should be maintained during transport and imaging.6 A healthcare provider capable of recognizing and responding to deterioration should stay with the patient and have the requisite equipment and medications. A head CT will delineate the presence and type of acute injury to the brain, thereby directing subsequent management. If the situation arises in which resuscitation priorities preclude transport to the radiology suite, neurosurgery should be consulted immediately.6 In addition to providing critical information early in the care of severe TBI, the non-contrast head CT has prognostic value.77,93-96
MRI is not standard in the early management of severe TBI because of the lack of universal availability, the time needed for imaging, and the difficulties with monitoring.92 MRI is superior to CT for evaluating non-hemorrhagic lesions such as DAI and the secondary effects of trauma such as ischemic injury.92,97 Plain radiographs of the skull have limited utility. They will reveal evidence of fracture but do not provide sufficient intracranial detail to direct further management. CT and MR-angiography play little role in the management of blunt TBI but can be used to evaluate vascular injury in penetrating trauma.92
Imaging of the cervical spine should be obtained in all patients with severe TBI. Plain films historically have been used for this purpose, but CT should be considered given the critical nature of the victim's injury, the efficiency (the patient will already be in the CT scanner), and the recent literature showing the superiority of CT over plain radiographs to detect bony injury.77,98-101 Neither CT nor plain radiographs of the cervical spine identify soft-tissue injuries adequately to clear the cervical spine in the acute phase of injury. In the absence of a fracture, cervical spine precautions should be maintained until the patient is cleared by established protocol. Imaging of the chest, abdomen, and extremities often will be required in patients with severe TBI and should be considered.
CT Findings. Acute blood appears as high-attenuation. Traumatic intracranial hemorrhages are categorized as epidural hematomas (EDH), intraparenchymal hemorrhages, subarachnoid hemorrhages (SAH), and subdural hematomas (SDH). (See Figure 2.) An EDH is a collection of blood between the dura mater and the skull. It is primarily a condition of the young and accounts for 0.5-1% of all TBI patients.102 EDHs are less frequent in the very young (< 2 years) and in the elderly because of the close attachment of the dura to the skull.12 EDH classically occurs from a direct blow to the head (e.g. assault, fall on object, or motor vehicle collision) resulting in skull fracture and rupture of the middle meningeal artery. As such, EDHs in the temporal or lateral frontopareital region should be considered arterial and immediately life-threatening.6 Epidural blood also can be from venous or fracture bleeding.6 EDHs are classically lenticular in shape, smooth, and may cross the midline.6 They are associated with a lucid interval, but this occurs less than 50% of the time.6,103 The underlying brain parenchyma tends to be minimally interrupted, and the lesions are amenable to rapid surgical repair.104 EDHs greater than 30 cm3 in volume should be evacuated surgically regardless of the patient's GCS score.105
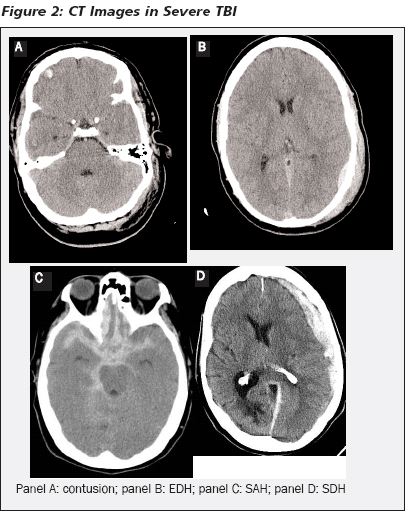 |
An intraparenchymal hemorrhage is an accumulation of blood within the brain parenchyma or the surrounding meningeal spaces. Acute cerebral contusions appear as focal areas of high attenuation with scattered foci of low attenuation (uninjured tissue).6 The most common locations are the temporal lobes and the base of the frontal lobes because of contact with adjacent bony surfaces. Edema tends to develop gradually, which results in increased mass effect and subsequent delayed deterioration and/or death.6 Lesions in the temporal lobes are constrained by the bone of the middle cranial fossa, which focuses expansion toward the brainstem, increasing the risk of rapid herniation.6,106
Traumatic SAH commonly appears over the convexities, within the sylvian fissure, or in the basal cisterns. The blood is contained between the pia mater and arachnoid. The accumulation of blood in the subarachnoid space leads to elevated ICP and decreased cerebral perfusion. The quantity of subarachnoid blood is of prognostic significance, but it is unclear if this is a result of the blood itself or because the quantity of blood is a marker of parenchymal injury.107,108
An SDH lies beneath the dura mater. SDHs are distributed over the cortex, causing their appearance as a crescent shape with an irregular border. SDHs often extend beyond suture lines. They can arise from parenchymal bleeding and can be arterial, venous, or from rupture of the bridging cortical veins. They typically arise from acceleration-deceleration injuries and frequently are associated with underlying paranchymal injuries, which remarkably impact their outcome.6,12 The midline shift can be larger than the hematoma itself, suggesting underlying brain injury.109 Brain injury can also result as the hematoma creates direct pressure on adjacent tissue. SDHs are much more common than EDHs and occur in 12-29% of patients with severe TBI.110 Because of the associated brain injury, the delay in clinical signs, and the advanced age of the afflicted population, the mortality is much higher than with EDH. Guidelines recommend that acute SDHs with either a thickness greater than 10 mm or a midline shift greater than 5 mm be evacuated surgically regardless of the patient's GCS score.110
DAI is a result of shearing or rotational forces that occur when the head is accelerated and decelerated rapidly.77 The subsequent stretching of axons at the gray-white junction of the cerebral cortex results in their widespread and often spontaneous and irreversible damage.111 The physical disruption of the cytoskeleton precipitates a sequence of events that finally results in cell death.112,113 Shear injury can be difficult to detect by CT scan but may appear as widely distributed punctate areas of high attenuation in the white and deep nuclear matter. Cerebral edema appears as local or global loss of gray-white differentiation.6
References
1. Langlois JA, Rutland-Brown W, Thomas KE. Traumatic brain injury in the United States: Emergency department visits, hospitalizations, and deaths. Atlanta: Centers for Disease Control and Prevention, National Center for Injury Prevention and Control; 2006.
2. Centers for Disease Control and Prevention. Traumatic Brain Injury. Available at: http://www.cdc.gov/ncipc/factsheets/tbi.htm Accessed February 10, 2009.
3. Kraus J, McArthur D. Epidemiology of Brain Injury. In: Cooper P, Golfinos J, eds. Head Injury. 4th ed. New York: McGraw-Hill Publishers; 2000:1-26.
4. Kochanek PM, Berger RP, Margulies SS, et al. Inflicted childhood neurotrauma: New insight into the detection, pathobiology, prevention, and treatment of our youngest patients with traumatic brain injury. J Neurotrauma. 2007;24(1):1-4.
5. Naval Medical Research Center. Operational Medicine Program. Available at: http://www.med.navy.mil/sites/nmrc/Pages/ccc_rm.htm. Accessed June 11, 2009.
6. Chesnut RM. Care of central nervous system injuries. Surg Clin North Am 2007; 87:119-156, vii.
7. Novack T. TBI Inform: Introduction to Brain Injury Facts and Stats. Issue 2. The University of Alabama at Birmingham. Available at: http://main.uab.edu/tbi/ show.asp?durki=27492&site=2988&return=57898. Accessed June 11, 2009.
8. Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet 1974;2:81-84.
9. Balestreri M, Czosnyka M, Chatfield DA, et al. Predictive value of Glasgow Coma Scale after brain trauma: Change in trend over the past ten years. J Neurology, Neurosurgery, Psychiatry 2004;75:161-162.
10. Stocchetti N, Pagan F, Calappi E, et al. Inaccurate early assessment of neurological severity in head injury. J Neurotrauma 2004;21:1131-1140.
11. Servadei F, Teasdale G, Merry G. Defining acute mild head injury in adults: A proposal based on prognostic factors, diagnosis, and management. J Neurotrauma 2001;18: 657-664.
12. Heegaard W, Biros M. Traumatic brain injury. Emerg Med Clin North Am 2007; 25:655-678, viii.
13. Chesnut RM, Marshall LF, Klauber MR, et al. The role of secondary brain injury in determining outcome from severe head injury. J Trauma 1993;34:216-222.
14. Pigula FA, Wald SL, Shackford SR, et al. The effect of hypotension and hypoxia on children with severe head injuries. J Pediatr Surg 1993;28:310-314; discussion 315-316.
15. Jones PA, Andrews PJ, Midgley S, et al. Measuring the burden of secondary insults in head-injured patients during intensive care. J Neurosurg Anesthesiol 1994;6:4-14.
16. Warner KJ, Bulger EM. Does pre-hospital ventilation effect outcome after significant brain injury? Trauma 2007;9:283-289.
17. van Hulst RA, Haitsma JJ, Lameris TW, et al. Hyperventilation impairs brain function in acute cerebral air embolism in pigs. Intensive Care Med 2004;30:944-950.
18. Sheinberg M, Kanter MJ, Robertson CS, et al. Continuous monitoring of jugular venous oxygen saturation in head-injured patients. J Neurosurg 1992;76:212-217.
19. Muizelaar JP, van der Poel HG, Li ZC, et al. Pial arteriolar vessel diameter and CO2 reactivity during prolonged hyperventilation in the rabbit. J Neurosurg 1988; 69:923-927.
20. Manley GT, Hemphill JC, Morabito D, et al. Cerebral oxygenation during hemorrhagic shock: Perils of hyperventilation and the therapeutic potential of hypoventilation. J Trauma 2000;48:1025-1032; discussion 1032-1023.
21. Rockswold G. Head Injury. In: Tintinalli J, ed. Emergency Medicine. New York: McGraw-Hill; 1996:913-921.
22. Zwienenberg M, Muizelaar JP. Vascular aspects of severe head injury. In: Miller L, Hayes R, eds. Head Trauma: Basic, Preclinical, and Clinical Directions. 1st ed. New York: Wiley-Liss; 2001:303-326.
23. Marmarou A, Signoretti S, Fatouros PP, et al. Predominance of cellular edema in traumatic brain swelling in patients with severe head injuries. J Neurosurg 2006;104: 720-730.
24. Vukic M, Negovetic L, Kovac D, et al. The effect of implementation of guidelines for the management of severe head injury on patient treatment and outcome. Acta Neurochir (Wien) 1999;141:1203-1208.
25. Palmer S, Bader MK, Qureshi A, et al. The impact on outcomes in a community hospital setting of using the AANS traumatic brain injury guidelines. American Associations for Neurologic Surgeons. J Trauma 2001;50:657-664.
26. Lu J, Marmarou A, Choi S, Maas A, et al. Mortality from traumatic brain injury. Acta Neurochirurgica 2005;95:281-285.
27. Hesdorffer DC, Ghajar J, Iacono L. Predictors of compliance with the evidence-based guidelines for traumatic brain injury care: A survey of United States trauma centers. J Trauma 2002;52:1202-1209.
28. Ghajar J, Hariri RJ, Narayan RK, et al. Survey of critical care management of comatose, head-injured patients in the United States. Crit Care Med 1995;23:560-567.
29. Fakhry SM, Trask AL, Waller MA, et al. Management of brain-injured patients by an evidence-based medicine protocol improves outcomes and decreases hospital charges. J Trauma 2004;56:492-499; discussion 499-500.
30. Vitaz TW, McIlvoy L, Raque GH, et al. Development and implementation of a clinical pathway for severe traumatic brain injury. J Trauma 2001;51:369-375.
31. Guidelines for the management of severe traumatic brain injury. J Neurotrauma 2007;24 Suppl 1:S1-106.
32. Brain Trauma Foundation. About BTF. Available at: http://www.braintrauma.org/site/PageServer?pagename=About_BTF. Accessed June 15, 2009.
33. Faul M, Wald MM, Rutland-Brown W, et al. Using a cost-benefit analysis to estimate outcomes of a clinical treatment guideline: Testing the Brain Trauma Foundation guidelines for the treatment of severe traumatic brain injury. J Trauma 2007;63: 1271-1278.
34. Badjatia N, Carney N, Crocco TJ, et al. Guidelines for prehospital management of traumatic brain injury 2nd edition. Prehosp Emerg Care 2008;12 Suppl 1:S1-52.
35. Baxt WG, Moody P. The impact of advanced prehospital emergency care on the mortality of severely brain-injured patients. J Trauma 1987;27:365-369.
36. Moppett IK. Traumatic brain injury: Assessment, resuscitation and early management. Br J Anaesth 2007;99:18-31.
37. McHugh GS, Engel DC, Butcher I, et al. Prognostic value of secondary insults in traumatic brain injury: Results from the IMPACT study. J Neurotrauma 2007; 24:287-293.
38. Silverston P. Pulse oximetry at the roadside: A study of pulse oximetry in immediate care. BMJ 1989;298:711-713.
39. Stocchetti N, Furlan A, Volta F. Hypoxemia and arterial hypotension at the accident scene in head injury. J Trauma 1996;40: 764-767.
40. Winchell RJ, Hoyt DB. Endotracheal intubation in the field improves survival in patients with severe head injury. Trauma Research and Education Foundation of San Diego. Arch Surg 1997;132:592-597.
41. Rudehill A, Bellander BM, Weitzberg E, et al. Outcome of traumatic brain injuries in 1,508 patients: Impact of prehospital care. J Neurotrauma 2002;19:855-868.
42. Resuscitation Outcomes Consortium. NIH hypertonic saline study update. May 12, 2009. Available at: https://roc.uwctc.org/tiki/tiki-index.php. Accessed June 16, 2009.
43. Patel HC, Bouamra O, Woodford M, et al. Trends in head injury outcome from 1989 to 2003 and the effect of neurosurgical care: An observational study. Lancet 2005;366:1538-1544.
44. Maas AI, Stocchetti N, Bullock R. Moderate and severe traumatic brain injury in adults. Lancet Neurology 2008;7: 728-741.
45. Varelas PN, Conti MM, Spanaki MV, et al. The impact of a neurointensivist-led team on a semiclosed neurosciences intensive care unit. Crit Care Med 2004;32: 2191-2198.
46. Visca A, Faccani G, Massaro F, et al. Clinical and neuroimaging features of severely brain-injured patients treated in a neurosurgical unit compared with patients treated in peripheral non-neurosurgical hospitals. Br J Neurosurg 2006;20:82-86.
47. Suarez JI, Zaidat OO, Suri MF, et al. Length of stay and mortality in neurocritically ill patients: Impact of a specialized neurocritical care team. Crit Care Med 2004;32:2311-2317.
48. Trunkey DD. Trauma centers and trauma systems. JAMA 2003;289:1566-1567.
49. MacKenzie EJ, Rivara FP, Jurkovich GJ, et al. A national evaluation of the effect of trauma-center care on mortality. N Engl J Med 2006;354:366-378.
50. Heros RC. Case volume and mortality. J Neurosurg 2003;99:805-806; discussion 806.
51. Davis DP, Hoyt DB, Ochs M, et al. The effect of paramedic rapid sequence intubation on outcome in patients with severe traumatic brain injury. J Trauma 2003;54: 444-453.
52. Klemen P, Grmec S. Effect of pre-hospital advanced life support with rapid sequence intubation on outcome of severe traumatic brain injury. Acta Anaesthesiol Scand 2006;50:1250-1254.
53. Wang HE, Yealy DM. Out-of-hospital rapid-sequence intubation for trauma patients. J Trauma 2004;56:722-723.
54. Davis DP, Peay J, Sise MJ, et al. The impact of prehospital endotracheal intubation on outcome in moderate to severe traumatic brain injury. J Trauma 2005;58: 933-939.
55. Warner KJ, Cuschieri J, Copass MK, et al. The impact of prehospital ventilation on outcome after severe traumatic brain injury. J Trauma 2007;62:1330-1336; discussion 1336-1338.
56. Davis DP, Stern J, Sise MJ, et al. A follow-up analysis of factors associated with head-injury mortality after paramedic rapid sequence intubation. J Trauma 2005;59: 486-490.
57. Davis DP, Heister R, Poste JC, et al. Ventilation patterns in patients with severe traumatic brain injury following paramedic rapid sequence intubation. Neurocritical Care 2005;2:165-171.
58. Dunford JV, Davis DP, Ochs M, et al. Incidence of transient hypoxia and pulse rate reactivity during paramedic rapid sequence intubation. Ann Emerg Med 2003;42:721-728.
59. Davis DP, Idris AH, Sise MJ, et al. Early ventilation and outcome in patients with moderate to severe traumatic brain injury. Crit Care Med 2006;34:1202-1208.
60. Davis DP, Douglas DJ, Koenig W, et al. Hyperventilation following aero-medical rapid sequence intubation may be a deliberate response to hypoxemia. Resuscitation 2007;73:354-361.
61. Davis DP, Fakhry SM, Wang HE, et al. Paramedic rapid sequence intubation for severe traumatic brain injury: perspectives from an expert panel. Prehosp Emerg Care 2007;11:1-8.
62. Davis DP. Early ventilation in traumatic brain injury. Resuscitation 2008;76: 333-340.
63. Bulger EM, Copass MK, Sabath DR, et al. The use of neuromuscular blocking agents to facilitate prehospital intubation does not impair outcome after traumatic brain injury. J Trauma 2005;58:718-723; discussion 723-714.
64. Davis DP, Peay J, Serrano JA, et al. The impact of aeromedical response to patients with moderate to severe traumatic brain injury. Ann Emerg Med 2005;46:115-122.
65. Citerio G, Vascotto E, Villa F, et al. Induced abdominal compartment syndrome increases intracranial pressure in neurotrauma patients: A prospective study. Crit Care Med 2001;29:1466-1471.
66. Bloomfield GL, Ridings PC, Blocher CR, et al. A proposed relationship between increased intra-abdominal, intrathoracic, and intracranial pressure. Crit Care Med 1997;25:496-503.
67. Aufderheide TP. The problem with and benefit of ventilations: Should our approach be the same in cardiac and respiratory arrest? Curr Opin Crit Care 2006;12: 207-212.
68. Muizelaar JP, Marmarou A, Ward JD, et al. Adverse effects of prolonged hyperventilation in patients with severe head injury: A randomized clinical trial. J Neurosurg 1991; 75:731-739.
69. Thomas SH, Orf J, Wedel SK, et al. Hyperventilation in traumatic brain injury patients: inconsistency between consensus guidelines and clinical practice. J Trauma 2002;52:47-52; discussion 52-43.
70. 2005 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. (page IV, 51-55). American Heart Association. Available at: www.circulationaha.org. Accessed June 16, 2009.
71. Yosefy C, Hay E, Nasri Y, et al. End tidal carbon dioxide as a predictor of the arterial PCO2 in the emergency department setting. Emerg Med J 2004;21:557-559.
72. Prause G, Hetz H, Lauda P, et al. A comparison of the end-tidal-CO2 documented by capnometry and the arterial pCO2 in emergency patients. Resuscitation 1997;35: 145-148.
73. Belpomme V, Ricard-Hibon A, Devoir C, et al. Correlation of arterial PCO2 and PETCO2 in prehospital controlled ventilation. Am J Emerg Med 2005;23:852-859.
74. Gennarelli TA, Champion HR, Sacco WJ, et al. Mortality of patients with head injury and extracranial injury treated in trauma centers. J Trauma 1989;29:1193-1201; discussion 1201-1192.
75. Siegel JH. The effect of associated injuries, blood loss, and oxygen debt on death and disability in blunt traumatic brain injury: The need for early physiologic predictors of severity. J Neurotrauma 1995;12:579-590.
76. ATLS: Advanced Trauma Life Support for Doctors, 7th ed. Chicago: American College of Surgeons; 2004.
77. Bhat R, Hudson R, Sabzevari T. An evidence-based approach to severe traumatic brain injury. Emerg Med Practice 2008;10:1-24.
78. Braakman R, Gelpke GJ, Habbema JD, et al. Systematic selection of prognostic features in patients with severe head injury. Neurosurgery 1980;6:362-370.
79. Fearnside MR, Cook RJ, McDougall P, et al. The Westmead Head Injury Project outcome in severe head injury. A comparative analysis of pre-hospital, clinical and CT variables. Br J Neurosurg 1993;7:267-279.
80. Narayan RK, Greenberg RP, Miller JD, et al. Improved confidence of outcome prediction in severe head injury. A comparative analysis of the clinical examination, multimodality evoked potentials, CT scanning, and intracranial pressure. J Neurosurg 1981;54:751-762.
81. Marion DW, Carlier PM. Problems with initial Glasgow Coma Scale assessment caused by prehospital treatment of patients with head injuries: Results of a national survey. J Trauma 1994;36:89-95.
82. Winkler JV, Rosen P, Alfry EJ. Prehospital use of the Glasgow Coma Scale in severe head injury. J Emerg Med 1984;2:1-6.
83. Bazarian JJ, Eirich MA, Salhanick SD. The relationship between pre-hospital and emergency department Glasgow coma scale scores. Brain Inj 2003;17:553-560.
84. Gill MR, Reiley DG, Green SM. Interrater reliability of Glasgow Coma Scale scores in the emergency department. Ann Emerg Med 2004;43:215-223.
85. Teoh LS, Gowardman JR, Larsen PD, et al. Glasgow Coma Scale: Variation in mortality among permutations of specific total scores. Intensive Care Med 2000;26:157-161.
86. Davis DP, Vadeboncoeur TF, Ochs M, et al. The association between field Glasgow Coma Scale score and outcome in patients undergoing paramedic rapid sequence intubation. J Emerg Med 2005;29:391-397.
87. Wijdicks EF, Bamlet WR, Maramattom BV, et al. Validation of a new coma scale: The FOUR score. Ann Neurology 2005;58: 585-593.
88. Stead LG, Wijdicks EF, Bhagra A, et al. Validation of a new coma scale, the FOUR score, in the emergency department. Neurocritical Care 2009;10:50-54.
89. Corrigan JD. Substance abuse as a mediating factor in outcome from traumatic brain injury. Arch Physical Med Rehab 1995;76: 302-309.
90. Brady WJ, Butler K, Fines R, et al. Hypoglycemia in multiple trauma victims. Am J Emerg Med 1999;17:4-5.
91. Britt P, Heiserman J. Imaging Evaluation. In: Cooper P, Golfinos J, eds. Head Injury. New York: McGraw-Hill; 2000:27-62.
92. Davis PC; Expert Panel on Neurologic Imaging. Head trauma. AJNR Am J Neuroradiol 2007;28:1619-1621.
93. van Dongen KJ, Braakman R, Gelpke GJ. The prognostic value of computerized tomography in comatose head-injured patients. J Neurosurg 1983;59:951-957.
94. Holliday PO, 3rd, Kelly DL, Jr., Ball M. Normal computed tomograms in acute head injury: Correlation of intracranial pressure, ventricular size, and outcome. Neurosurgery 1982;10:25-28.
95. Lobato RD, Cordobes F, Rivas JJ, et al. Outcome from severe head injury related to the type of intracranial lesion. A computerized tomography study. J Neurosurg 1983; 59:762-774.
96. Gennarelli TA, Spielman GM, Langfitt TW, et al. Influence of the type of intracranial lesion on outcome from severe head injury. J Neurosurg 1982;56:26-32.
97. Gentry LR. Imaging of closed head injury. Radiology 1994;191:1-17.
98. Barba CA, Taggert J, Morgan AS, et al. A new cervical spine clearance protocol using computed tomography. J Trauma 2001; 51:652-656; discussion 656-657.
99. Diaz JJ Jr., Gillman C, Morris JA Jr., et al. Are five-view plain films of the cervical spine unreliable? A prospective evaluation in blunt trauma patients with altered mental status. J Trauma 2003;55:658-663; discussion 663-654.
100. Schenarts PJ, Diaz J, Kaiser C, et al. Prospective comparison of admission computed tomographic scan and plain films of the upper cervical spine in trauma patients with altered mental status. J Trauma 2001;51:663-668; discussion 668-669.
101. Widder S, Doig C, Burrowes P, et al. Prospective evaluation of computed tomographic scanning for the spinal clearance of obtunded trauma patients: Preliminary results. J Trauma 2004;56:1179-1184.
102. Greenberg M. Handbook of Neurosurgery. Lakeland (FL): Greenberg Graphics; 1997.
103. Chiles B, Cooper P. Extra-axial hematomas. In: Loftus C, ed. Neurosurgical Emergencies. Park Ridge (IL): American Academy of Neurosurgeons; 1994:73-100.
104. Munch E, Horn P, Schurer L, et al. Management of severe traumatic brain injury by decompressive craniectomy. Neurosurgery 2000;47:315-322; discussion 322-313.
105. Bullock MR, Chesnut R, Ghajar J, et al. Surgical management of acute epidural hematomas. Neurosurgery 2006;58(3 Suppl):S7-15; discussion Si-iv.
106. Oertel M, Kelly DF, McArthur D, et al. Progressive hemorrhage after head trauma: Predictors and consequences of the evolving injury. J Neurosurg 2002;96:109-116.
107. Bullock R, Chesnut RM, Clifton G, et al. Guidelines for the management of severe head injury. Brain Trauma Foundation. Eur J Emerg Med 1996;3:109-127.
108. Servadei F, Murray GD, Teasdale GM, et al. Traumatic subarachnoid hemorrhage: Demographic and clinical study of 750 patients from the European brain injury consortium survey of head injuries. Neurosurgery 2002;50:261-267; discussion 267-269.
109. Zumkeller M, Behrmann R, Heissler HE, et al. Computed tomographic criteria and survival rate for patients with acute subdural hematoma. Neurosurgery 1996;39: 708-712; discussion 712-703.
110. Bullock MR, Chesnut R, Ghajar J, et al. Surgical management of acute subdural hematomas. Neurosurgery 2006;58(3 Suppl):S16-24; discussion Si-iv.
111. Arundine M, Aarts M, Lau A, et al. Vulnerability of central neurons to secondary insults after in vitro mechanical stretch. J Neurosci 2004;24:8106-8123.
112. Iwata A, Stys PK, Wolf JA, et al. Traumatic axonal injury induces proteolytic cleavage of the voltage-gated sodium channels modulated by tetrodotoxin and protease inhibitors. J Neurosci 2004;24: 4605-4613.
113. Wolf JA, Stys PK, Lusardi T, et al. Traumatic axonal injury induces calcium influx modulated by tetrodotoxin-sensitive sodium channels. J Neurosci 2001;21: 1923-1930.
This is the first of a two-part series on severe traumatic brain injury, focusing on the evidence for optimal care.Subscribe Now for Access
You have reached your article limit for the month. We hope you found our articles both enjoyable and insightful. For information on new subscriptions, product trials, alternative billing arrangements or group and site discounts please call 800-688-2421. We look forward to having you as a long-term member of the Relias Media community.
