Missed Myocardial Infarction: ECG Strategies to Reduce the Risk
Missed Myocardial Infarction: ECG Strategies to Reduce the Risk
Does the electrocardiograph in your emergency department include a computer-generated interpretation on every electrocardiogram printed? Like me, do you often find them less than helpful? Occasionally troubling? How do you react when the computer says "consistent with acute ischemia" and the patient is perfectly fine? As in most situations in medicine, the utility of any ancillary test requires judgment within the clinical context. So it is with ECGs. While getting better, computer analysis of chest wall voltages cannot replace the human analysis of the entire patient, including the ECG. That said, studies indicate that emergency physicians do miss important ECG patterns. This issue of EM Reports covers important concepts in the ECG diagnosis of myocardial infarction.
J. Stephan Stapczynski, MD, Editor
ECG Interpretation
Of clinical features useful in MI diagnosis, the ECG is the most important bedside finding to diagnose acute MI.1 The ECG is the branch point in treatment of acute MI, as patients with STEMI are taken for emergent reperfusion therapy, and those with non-STEMI are treated medically. The ECG also gives data on the location and extent of injury. (See Table 1.)
 |
Several studies have found that the sensitivity of the initial ECG for detection of acute MI can be as low as 50%.2 Specificity also can be less than desired, as noted from a recent study in which 15 experienced cardiologists were given 116 ECGs to evaluate; percutaneous coronary intervention was recommended in 7.8-33% of cases even though only 8% actually had STEMI.3 Two other important observations are that a normal ECG is present in up to 4% of acute MI cases,4 and patients having acute MI who present with normal or non-diagnostic ECGs are twice as likely to present atypically, i.e. without chest pain as well (11-14% vs. 24%).5
Patients with initially non-diagnostic ECGs often will develop ECG findings of MI in the first few hours after hospital arrival.6 Thus, the importance of a second ECG or continuous ST segment monitoring to help detect the evolving acute MI in the ED. (See Figure 1.) Depending on the clinical situation, ECGs can be repeated every 30 minutes to 4 hours.7
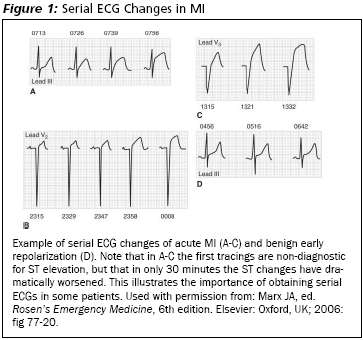 |
Prior studies on missed MI in the ED found that 25% of these patients were discharged with ST segment elevation on their ECG.8 More recent work by Pope in 2000 found 11% of missed MI patients were still being discharged with ST segment elevations of 1-2 mm on their ECGs.9 The most common misdiagnoses of ST segment elevation on the ECG were left ventricular aneurysm, benign early repolarization, and left ventricular hypertrophy.10 There are several useful principles on interpretation of difficult ECGs. (See Table 2.)
 |
ECG Criteria for Diagnosis of MI
STEMI. The ECG criteria for STEMI diagnosis, are > 1 mm ST segment elevation in two contiguous leads for limb leads (I, II, III, aVF, aVL) and > 2 mm ST segment elevation in precordial leads (V1-V6). Posterior infarcts are the major exception to this. The ST segment elevation can be rather variable in appearance. (See Figure 2.) In most cases of MI, the actual slope is flat or concave upward. In some cases the slope is downward (convex), which can be confused with early repolarization. While ST segment elevation is the typical finding in STEMI, the earliest changes are hyperacute T waves or T waves that are much higher and wider than normal. (See Figure 3.) These are seen most often in anterior MIs (leads V2-V5).
 |
 |
STEMI result from larger, more proximal blockages in the major coronary vessels (right, LAD, circumflex). Occlusion of these vessels causes a full thickness ischemia of a portion of the heart. The ST segments "elevate" on the ECG is because ischemia changes the electrical properties (elevated resting membrane potential, lengthens action potential) of the affected myocytes. These changes produce a voltage gradient between normal and ischemic regions (the injury current). (See Figure 4.) Likewise, ST segment depressions (reciprocal changes) also are seen in STEMIs, as this represents the same voltage differences seen on lead sensing the infarct from the opposite side of the heart. For example, ST segment elevation with anterior MI will produce inferior ST segment depression. Reciprocal changes from an inferior MI are best seen in lead aVL. For reasons that are not well understood, reciprocal changes are only seen in about 80% of inferior MIs, and only 33% of anterior MIs.10,11 The ST segment depression of posterior MIs actually represents the reciprocal changes from elevation present leads V8-V9 (when additional leads are used). The presence of reciprocal changes increases the likelihood of acute MI with a positive predictive value of > 90%.12
 |
Natural Evolution of ECG Changes in STEMI. The ECG undergoes predictable changes during the course of an untreated STEMI. The very first signs on the ECG are hyperacute T waves (see Figure 3), which appear in the first few minutes after vessel occlusion. However, they are transient and are not often seen because most patients usually have not arrived at the hospital. Following a variable period of time (hours to days), the ST segments will return to the normal baseline. Up to 95% of inferior but only 40% of anterior ST segment elevations are resolved in 2 weeks.13 Persistence of elevation for more than 2 weeks is associated with greater morbidity, as 60% of these patients develop a ventricular aneurysm.13
Later in the course of a STEMI when the ST segments are no longer elevated, deep T wave inversions usually appear in the leads where the ST segments were elevated, sometimes as early as 72 hours after MI. The T wave inversions can resolve over time (days, weeks, even months) or remain permanent. Q waves also appear in these leads, but are not always seen in all STEMIs. They can develop in as little as 1-2 hours after onset of symptoms or may take 8-12 hours.12 Q waves also can be produced by some subendocardial (non-transmural) infarct. The presence of Q waves is not always pathologic and up to 12% of healthy young men can have inferior Q waves.14 Normal Q waves are narrow with a duration of less than 0.04 seconds, and of low amplitude of less than one-third the size of the accompanying R wave.
Non-STEMI. In non-STEMI, the area of infarcting muscle is smaller, in most cases because a smaller or branch coronary vessel is occluded instead of a primary vessel. Subendo-cardial ischemia/infarction also can occur without vessel occlusion because this area of the heart is the most distant from coronary supply. When chronic hypertension leads to a thickened left ventricle, the combination of increased blood pressure and increased distance from coronary flow can produce areas of ischemia without vessel occlusion. The ECG shows ST segment depression instead of elevation. In some cases, deep T wave inversions are seen instead of ST segment depression, which is thought to be due more to ischemia (unstable angina) than infarction. The term Wellen's syndrome is used to describe deep T wave inversions in leads V1-V4, and this pattern correlates with LAD stenosis.15
Non-ischemic conditions also can cause ST segment depression, including LVH, left or right bundle branch blocks, digitalis effect, hypokalemia, and cardiomyopathy.
ECG Diagnosis of MIClassic Patterns
In patients with normal coronary anatomy, the infarct-related vessel's location on the heart will produce typical changes on the ECG. (See Table 1.) (See Table 3.)
 |
Anterior MI. Anterior STEMI is produced by a thrombus in the left anterior descending artery (LAD), which supplies the anterior surface of the left ventricle. ST segment elevations are seen in leads V2-V4, with reciprocal changes in inferior leads (II, III, and aVF). The elevations can be quite large (4-6 mm or more) and have a rounded appearance. The rounded appearance and increased mortality associated with anterior MI gives the term "tombstones" for the ST segment elevations. Variations on this pattern include anterior-lateral MI, anterior-septal MI, and anterio-inferior MI. Lateral involvement is seen when elevations include leads V5-V6, and septal extension is noted with elevation in V1-V2 primarily. When the LAD wraps around the apex of the heart, it supplies the inferior wall (typically territory of the right coronary artery), and therefore ST segment elevation may be seen in inferior leads instead of reciprocal depression.
Anterior STEMIs can be more subtle. One example is when an RBBB is produced by occlusion of the first septal branch off the LAD. The patient will have a new RBBB, which can make the ST segment elevations much more difficult to see. (See Figure 5.) Another example is when the ECG is taken early in the course of the MI and only hyperacute T waves are present. (See Figure 3.) If the ECG is not repeated, the later ST elevation will be missed.
 |
Inferior MI. In the majority of cases (80-90%), inferior STEMI occurs by thrombus in the right coronary artery.16 The right coronary is said to be dominant, i.e., supplies the right ventricle and 20-25% of the left ventricle, in about 80-90% of people. In the remainder of people, the circumflex is dominant and wraps around the apex of the heart to supply the right ventricle as well. Therefore, a clot in the circumflex also can produce inferior STEMI changes.
ST segment elevations are easiest to see in lead III followed by lead aVF and lead II. Reciprocal depression usually is seen in lead aVL, and if this is absent, the diagnosis of acute inferior MI should be reconsidered. ST depression also seen in V1-V4 is indicative of posterior wall injury. Bradycardia is common in inferior MI due to ischemia of the AV node.
ST segment elevation in V1-V3 with inferior MI suggests right ventricle involvement, which occurs in about 30% of inferior MIs.16 Right ventricular involvement almost always is due to occlusion of the right coronary proximal to the RV branch.17 Rarely, RV infarction can occur from occlusion of a dominant left circumflex that supplies the right ventricle as well. One recent study suggests the possibility of using ST depression in lead aVL as a sign of RV infarction,18 finding that > 1 mm ST depression in aVL was 87% sensitive, 91% specific, with a diagnostic accuracy of 89% for detecting right ventricle infarction among inferior MI patients.18
Ultimately, right ventricle MI is confirmed by taking a right-sided ECG and finding ST elevation in V4R. ST elevation in V4R has a sensitivity and specificity of > 90% for right ventricle infarctions.19 However, ST elevation in V4R is often transient in inferior STEMI, may be very subtle (just 1 mm), and may fade quickly after only a few hours.
Inferior STEMI can be difficult to detect because the degree of ST elevation can vary from 8 mm to barely 1 mm. Comparison with old ECGs when available may help highlight these subtle changes, and one also can obtain serial ECGs more frequently (every 10-15 minutes) to look for evolution to more obvious STEMI findings.
Lateral MI. Isolated lateral STEMI manifests with ST segment elevation in V5, V6, aVL, and lead I, and usually results from blockage of the left circumflex artery. Lateral MIs also can appear simultaneously with posterior MI or as posterior/inferior MI with occlusion of a dominant circumflex. Blockage of a dominant right coronary also will give an inferior/posterior/lateral MI pattern. The ECG is less sensitive for detecting lateral MIs than anterior or inferior MIs. ST elevation in lateral leads is generally small (> 2 mm in only 5%).20 Only 66% have both ST elevation and reciprocal depression, 30% have isolated ST depression, and 33% have neither ST elevation nor depression.21 Although not common, about 10% of lateral MIs also will have right ventricle infarction from occlusion of a dominant circumflex.17
Difficult-to-Detect MIs on ECG
Several MI patterns are more difficult to diagnose. They include posterior MI, MI in the presence of LBBB, and MI with a paced rhythm. (See Table 4.)
 |
Posterior MI. A posterior STEMI is unique in that it is the only STEMI that does not produce ST elevation on the standard 12-lead ECG. As noted, posterior MI will have reciprocal ST segment depression in leads V1-V4. The ST elevation from isolated posterior MI will be seen only when the 15-lead ECG is used, adding leads V7-V9. The additional leads are placed on the fifth intercostal space; V7 at the posterior axillary line, V8 at the scapular tip, and V9 at the left paraspinal border. The criteria for posterior MI is only 1 mm of ST elevation in these leads, not 2 mm as in precordial leads. Some even have suggested using 0.5 mm of ST elevation to increase sensitivity.22
Posterior MIs are much less common than other STEMIs, making up only 3-8% of STEMIs.23 The ECG pattern can be similar to that of anterior ischemia and, as expected, posterior MIs are missed much more often than other STEMIs.22 However, on close evaluation, the anterior ST segment depression in posterior MI often is upsloping compared to the downsloping segments in anterior ischemia. Normally, there also is an upright R wave and an upright T wave with posterior MI, whereas with anterior ischemia the T wave often is flipped. In addition, the ST changes in anterior ischemia often extend throughout precordial leads to include V6, whereas in posterior MI, V5 and V6 are not usually affected.
LBBB with MI. MI in the presence of LBBB is a well-known example of a difficult ECG diagnosis. Although LBBB is seen only in about 1% of the general population, the incidence increases with age, and roughly 6-9% of acute MI patients will have LBBBs.24 LBBB naturally produces ST-T wave changes that, as a general rule, mimic or hide the presence of acute MI. (See Insert Figure 6.) However, in some cases, STEMI still can be detected using the Sgarbossa criteria.25
 |
The Sgarbossa criteria consist of three ECG findings used to detect STEMI in the presence of LBBB. They are, in order of decreasing sensitivity, ST elevation of > 1 mm in more than one lead concordant (in the same direction) with the QRS complex; ST depression of > 1 mm in leads V1-V3; and ST elevation of > 5 mm discordant with the QRS complex. (See Insert Figure 6.) Concordant ST elevation should be present in the leads in which the QRS is predominantly positive (typically leads V5, V6, I, II, and aVL). More recent studies have found that discordant 5 mm ST elevation was seen in only 26% of patients with acute MI, and this finding alone may not be useful.26 However, the other two Sgarbossa criteria remained effective in a recent meta-analysis.27
RBBB with MI. The presence of a RBBB does not impede the diagnosis of acute MI nearly as much as an LBBB, but it still can be problematic as it may be difficult to see where the QRS complex ends and the ST segment begins. (See Figure 5.) One can determine the length of the QRS in unaffected leads and apply that duration to the lead in question. In STEMI patients with RBBB, the T wave inversion and ST segment depression normally seen in leads V1 to V3 due to the RBBB can hide the ST segment elevation due to the infarction.28
Paced Rhythm with MI. Standard right ventricle pacemakers produce an ECG similar in appearance to that of an LBBB and, as a result, acute MI is hard to diagnose in a paced rhythm. Leads V1-V3 in some patients can show conspicuous ST elevation simply as a result of the pacing. The Sgarbossa criteria can aid in detection of acute MI in paced rhythms.29 However, the Sgarbossa criteria in paced rhythms are not as accurate as for LBBB. The most accurate marker is discordant > 5 mm ST elevation most often seen in leads V2-V4.30 This was found to be only 53% sensitive, but had a higher specificity of 88%. Two other criteria were suggested and may be helpful in some cases, but were not found to be statistically significant.29 Some physicians suggest it may not be possible to accurately identify an acute MI with a ventricular paced rhythm.
ECG Patterns that Mimic STEMI
The other side of the coin in ECG diagnosis of acute MI is being aware of conditions that can simulate acute MI when it is not present. The sensitivity of ST elevation alone for acute MI is not particularly high, largely due to these other conditions that also produce ST changes. One series found that in ED patients with chest pain and ST elevation, only 15% were from acute MI while the other 85% were from non-MI diagnoses: 25% were from LVH, 15% from LBBB, 12% from early repolarization, and the remainder were from RBBB, LV aneurysm, pericarditis, with 17% undefined.31 Given the pressures on ED staff to reduce door-to-drug times32 and the pay-for-performance incentives in place at many hospitals to meet pre-set timelines in treatment of acute MI, one must be extra careful to keep the number of patients misdiagnosed with acute MI to a minimum as well. While these patients do not experience the same increased mortality rates as do MIs that are missed and sent home, they still may undergo unnecessary risk from invasive procedures and/or thrombolytic drugs.
The incidence of patients who are misdiagnosed with acute MI is unknown. One older study of 93 patients who were all given thrombolytic therapy for presumed acute MI found that 11% did not actually have an acute MI.33 Of these 9 patients, 30% instead had early repolarization, 30% had LVH, and the other 30% had intraventricular conduction delays (i.e., bundle branch blocks).33 A more recent study of 820 patients referred for urgent catheterization who initially were diagnosed with acute MI found that 2.3% were not having an MI.34 A third larger study of 1345 patients referred for emergent angioplasty found 14% had no coronary lesion identified.35
Benign Early Repolarization. Benign early repolarization (BER) is a variant of the normal ECG, seen in 1-5% of healthy adults, often in young men (< 40 years), especially athletes or those with natural bradycardia.36 It also has been associated with black men age 20-40 years, but some have disputed this connection.37 The exact basis of the changes seen in BER is not proven, but one theory is that they arise from an early repolarization of the subepicardium relative to the subendocardial area. As a result of the rapid repolarization, mild ST elevation occurs. The following criteria are used to define BER: ST elevation with upward concavity of the initial segment, notching or slurring of the terminal QRS complex (J point), symmetric and concordant large T waves, widespread or diffuse ST elevation, and temporal stability.38 (See Insert Figure 7.)
 |
Correct identification of BER is important in ED chest pain patients not only to keep from confusing this with ACS, but also because of the relatively high frequency with which it is seen in ED populations. Although only seen in 1-5% of the general population, several series have found anywhere from 13% to 48% of ED chest pain patients have BER on their ECG.39 One useful feature to identify BER is J point elevation. The J point refers to the junction where the QRS ends and the ST segment begins. In BER, J point elevation usually is < 3.5 mm, and the ST segment appears to be evenly lifted from the baseline, thus preserving the normal upward sloping initial ST segment shape.39 A J point that is notched or irregular also is suggestive of BER. The tall T waves always should be concordant with the QRS complex as well. ST segment elevation usually is present in leads V2-V5, and elevation in limb leads is less common. The up-sloping or concave ST segment is only a guideline, as the ST segment also can be concave early in acute MI.40
Left Ventricular Hypertrophy (LVH). LVH is a common finding on ECGs, seen in roughly 3% of men and 1.5% of women.40 ECG criteria for LVH include increased height of the QRS complex with tall R waves (11-13 mm in aVL) in leads I, aVL, V5, and V6 and deep S waves in leads V1 and V2. Altogether, 70% of patients with LVH changes on their ECG will have ST-T wave changes.41 ST segments can be elevated or depressed, and T waves can be tall or inverted. (See Insert Figure 8.) The abnormal repolarization changes in LVH naturally produce baseline ECG changes that can hide or mimic acute MI. One study found misinterpretation of the LVH changes was responsible for 70% of patients initially being misdiagnosed with ACS who later were found not to have acute MI.42
 |
There are two useful features one can use to distinguish acute MI from LVH in the ECG. First, the shape of the ST segment in LVH tends to be concave upward, while in acute MI it tends to be convex downward or flat. (See Figure 8.) The strain pattern of lateral ST depression with inverted T waves (strain pattern) often is seen in leads V3-V6 as a "mirror image" of the ST elevation in leads V1-V2. (See Insert Figure 8.) When present together, this combination can be reassuring that ECG changes are due to LVH and not acute MI.43 The second method is to use serial ECGs. The changes of LVH will not progress over the next several hours like those of an acute MI do.
Acute Pericarditis. Patients with acute pericarditis and ST elevation make up about 1% of ED patients with ST elevation.28 Their clinical presentation can either be with pleuritic chest pain or with pain similar to that of acute cardiac ischemia. Substernal chest pressure was the presenting complaint in 36% of patients with pericarditis in one recent series.44 Although not always present, the chest pain classically is worse when supine and improved when sitting up. Viral prodromes are noted in about 50% of patients, and up to 60% of patients present with fever.45 Patients also can present with dyspnea and have pericardial friction rubs. Cardiac biomarkers often are elevated in acute pericarditis. One recent study found that troponin I levels were elevated in 32% of patients with pericarditis but, unlike acute MI, higher troponin levels were not associated with worse outcomes.46
ECG changes seen in acute pericarditis can be confused with those of acute MI, and the distinction is important to avoid treating these patients with anticoagulants or thrombolytics that theoretically could induce bleeding in the pericardial space and lead to tamponade. Cardiac tamponade after systemic thrombolytics has been documented in large series to occur in about 1% of cases.47
The well-known ECG changes seen in pericarditis are those of diffuse ST segment elevation and PR segment depression. However, a variety of other ECG changes also can occur, including T wave inversions, ST segment depression, prolonged QT interval, bundle branch blocks, and even Q-waves.44 These changes result from injury currents caused by general irritation of the myocardial surface and can appear similar to STEMI on first glance. ECG findings in acute pericarditis also tend to evolve in four classic stages: ST elevation seen in Stage 1, resolution of ST elevation in Stage 2, T-wave inversions in Stage 3, and normalization of the ECG in Stage 4.44 These stages can occur in an unpredictable fashion. Some patients pass through Stages 1-3 in only a few hours, while others may take weeks to do so. Yet others will not exhibit all four stages and may progress directly from Stage 1 to Stage 4.
Fortunately, several useful clues can help differentiate acute pericarditis from STEMI. The single most useful finding is that of PR segment depression. PR depression arises from inflammation of the atria and is considered "almost diagnostic" of acute pericarditis.41 PR depression is best seen in leads V5, V6, II, III, and aVF, while reciprocal PR elevation can be seen in aVR and may be more obvious than PR depression.48 ST elevation usually is diffuse and essentially is seen in every lead except aVR and V1. Thus, the elevation overlaps the territory of all three coronary vessels, which is atypical for STEMI. Reciprocal ST depression also can occur in lead aVR. More focal ST elevation also can occur with more localized inflammation49,50 and, when present, is seen most often in inferior leads.44 ST elevation can vary from 1-4 mm, and is rarely > 5 mm. The ST segment most often is concave, but can be convex or flat as in elevation in acute MI. T wave inversion can follow the ST elevation as in acute MI and is seen in leads where ST elevation occurred.
In the absence of PR depression, the ECG alone may not distinguish between acute pericarditis and acute STEMI.
Persistent ST Elevation After MI (LV Aneurysm). In some cases, typically after a large transmural MI, ST segment elevation remains present for more than six months.51 The majority of cases with persistent ST elevation seem to follow anterior MI but also can develop on the inferior and posterior walls. In the past, persistent ST elevation on the ECG commonly was associated with an LV aneurysm, and even some recent studies found that 30% of patients with persistent elevation did show evidence of LV aneurysm by echocardiography.52 However, others suggest this is not the case.53
LV aneurysm is a complication that can follow large transmural MIs when a larger area of cardiac muscle dies and is replaced by a relatively weak, thin layer of necrotic muscle and fibrous scar that bulges out during systole. The LV aneurysms range from smaller ones only 1 cm in diameter to large ones up to 8 cm.53 Unlike pseudoaneurysms, which actually are contained myocardial wall ruptures, true cardiac aneurysms rarely lead to rupture. Even if aneurysm is not present, persistent ST elevation is associated with large areas of damage from acute MI. Only 10% of patients with anterior wall scars < 30% developed persistent ST elevation, but 78% of those with scars > 50% did.50 Thus, the risk of persistent ST elevation correlates with the size of the previous infarct, but the mechanism for the persistent elevation remains unclear.
In some patients, persistent ST elevation after MI can appear very similar to an acute anterior MI. In other cases, the ST segments only partially return to normal, and the T waves can stay inverted, giving the suggestion of a late presentation of acute MI. Three features can be used to help differentiate LV aneurysm from STEMI. First, reciprocal changes will not be present, and the expected evolution of the ST segments/T waves also will not occur. In addition, Q waves will be present in the same distribution as the elevation. The T wave amplitude/QRS amplitude did help distinguish LV aneurysm from acute MI.54 A ratio > 0.36 in any single lead likely was caused by acute MI, while a ratio < 0.36 in all leads was associated with LV aneurysm.54
Subarachnoid Hemorrhage. ECG changes occur in 60-70% of patients with intracerebral hemorrhage and 40-70% of those with subarachnoid hemorrhage (SAH).55 These changes also have been described less often with ischemic stroke and head trauma. Inverted T waves associated with prolonged QT intervals are the most common finding, but ST segment changes and large U waves occur as well.56 Deeply inverted T waves due to cardiac sources are associated with normal QT intervals, while those from stroke or SAH present with prolonged QT intervals.57 When ST segment elevations are present, regional wall motion defects not due to coronary disease are seen.56 These cardiac changes often spontaneously resolve over several days in patients who survive their CNS pathology. This recovery is similar to Takotsubo syndrome, and catecholamine release is hypothesized as a cause for both conditions.58 Elevated troponin also can be seen and, as expected, is associated with increased mortality.58
In most cases, the patient's history (chest pain vs. headache) would keep one from going astray, but patients who present with mental status changes may not be able to give this key information.
Brugada Syndrome. The Brugada syndrome is a genetic disorder with autosomal dominant transmission associated with sudden cardiac death.59 The patients have normal hearts and no coronary disease but have several abnormalities on their ECGs: an RBBB pattern with ST segment elevation on the right precordial leads.59 The syndrome has been traced to a defect in the cardiac sodium channel, which increases risk of spontaneous ventricular tachycardia/fibrillation.60 The sodium channel defect is not always evident, but can be worsened by higher body temperatures and type I antidysrhythymics.60 The syndrome also has been uncovered in patients with cocaine toxicity61 or fever.62 Brugada syndrome is associated with ethnic populations (e.g., Southeast Asians), where it is estimated to cause 40-50% of cases of spontaneous ventricular fibrillation63 and 4-12% of all sudden unexpected deaths. Although less common, Brugada syndrome also has been described in Caucasians and Hispanics.62,64
Brugada syndrome is relevant to this discussion because the ST elevation seen in the baseline ECG can simulate acute MI. (See Insert Figure 9.) More importantly, the Brugada pattern is not static and can be a transient finding64 perhaps leading to confusion with serial ECG interpretation. In many patients, the pattern can appear and completely disappear, or it can change between the two variants. (See Insert Figure 9.) Patients often self-terminate from their ventricular dysrhythmias and present only with syncope. The pattern often is first detected in young adults but also is seen in school-age children. Other than syncope or cardiac arrest due to ventricular fibrillation, no other symptoms are ascribable to Brugada syndrome. Therefore, the presence of ST elevation in leads V1-V3 in an otherwise asymptomatic patient should prompt concern for the diagnosis. Consultation with a cardiologist for assistance with ECG interpretation and disposition of the patient is recommended.
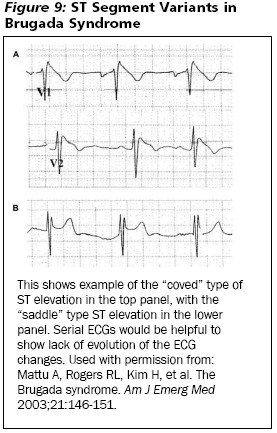 |
Conclusion
While important, the ECG is only an imperfect tool for the diagnosis of acute coronary syndrome. Understand its limitations and learn to use the ECG as an adjunct to, and not a replacement for, your clinical judgment.
References
1. Chun AA, McGee SR. Bedside diagnosis of coronary artery disease: A systematic review. Am J Med 2004;117:334-343.
2. Lee TH, Rouan GW, Weisberg MC, et al. Sensitivity of routine clinical criteria for diagnosing myocardial infarction within 24 hours of hospitalization. Ann Intern Med 1987;106:181-186.
3. Jayroe JB, Spodick DH, Nikus K, et al. Nonischemic causes of ST elevation by analyzing the presenting electrocardiogram. Am J Cardiol 2009;103:301-306.
4. Brady WJ, Roberts D, Morris F. The nondiagnostic ECG in the chest pain patient: Normal and nonspecific initial ECG presentations of acute MI. Am J Emerg Med 1999;17:394-407.
5. Welch RD, Zalenski RJ, Frederick PD, et al. Prognostic value of a normal or nonspecific initial electrocardiogram in acute myocardial infarction. JAMA 2001;286: 1977-1984.
6. Gibler WB, Sayre MR, Levy RC, et al. Serial 12-lead EKG monitoring in patients presenting to the emergency department with chest pain. J Electrocardiol 1993;26 Suppl:238-243
7. Velez J, Brady WJ, Perron AD, et al. Serial electrocardiography. Am J Emerg Med 2002;20:43-49.
8. McCarthy BD, Beshansky JR, D'Agostino RB, et al. Missed diagnoses of acute myocardial infarction in the emergency department: Results from a multicenter study. Ann Emerg Med 1993;22:579-582.
9. Pope JH, Aufderheide TP, Ruthazer R, et al. Missed diagnoses of acute cardiac ischemia in the ED. N Engl J Med 2000;342:1163-1170.
10. Brady WJ, Perron AD, Syverud SA, et al. Reciprocal ST segment depression: Impact on the electrocardiographic diagnosis of ST segment elevation acute myocardial infarction. Am J Emerg Med 2002;20:35-38.
11. Parale GP, Kulkarni PM, Khade SK, et al. Importance of reciprocal leads in acute myocardial infarction. J Assoc Physicians India 2004;52:376-379.
12. Brady WJ, Aufderheide TP, Chan T, et al. Electrocardiographic diagnosis of acute myocardial infarction. Emerg Med Clin North Am 2001;19:295-320.
13. Mills RM, Young E, Gorlin R, et al. Natural history of S-T segment elevation after acute myocardial infarction. Am J Cardiol 1975;35:609- 14.
14. Fisch C. Abnormal ECG in clinically normal individuals. JAMA 1983;250:1321-1323.
15. Tandy TK, Bottomy DP, Lewis JG. Wellen's syndrome. Ann Emerg Med 1999;33:347-351.
16. Atar S, Barbagelata A, Birnbaum Y. Electrocardiographic diagnosis of ST-elevation myocardial infarction. Cardiol Clin 2006;24:343-365.
17. Moye S, Carneya MJ, Holstege C, et al. The electrocardiogram in right ventricular myocardial infarction. Am J Emerg Med 2005;23:793-799.
18. Turhan H, Yilmaz MB, Yetkin E, et al. Diagnostic value of aVL derivation for right ventricular involvement in patients with acute inferior myocardial infarction. Ann Noninvasive Electrocardiol 2003;8:185-188.
19. Braat SH, Brugada P, den Dulk K, et al. Value of lead V4R for recognition of the infarct coronary artery in acute inferior myocardial infarction. Am J Cardiol 1984;53:1538-1541
20. Smith SW, Whitwam W. Acute coronary syndromes. Emerg Med Clin North Am 2006;24:53-89.
21. Veldkamp RF, Sawchak S, Pope JE, et al. Performance of an automated real-time ST segment analysis program to detect coronary occlusion and reperfusion. J Electrocardiol 1996;29:257-263.
22. Wung SF, Drew BJ. New electrocardiographic criteria for posterior wall acute myocardial ischemia validated by a percutaneous transluminal coronary angioplasty model of acute myocardial infarction. Am J Cardiol 2001;87:970-974.
23. Oraii S, Maleki I, Tavakolian AA, et al. Prevalence and outcome of ST segment elevation in posterior electrocardiographic leads during acute myocardial infarction. J Electrocardiol 1999;32:275-278.
24. Stenestrand U, Tabrizi F, Lindback J, et al. Comorbidity and myocardial dysfunction are the main explanations for the higher 1-year mortality in acute myocardial infarction with left bundle branch block. Circulation 2004;110:1896-1902.
25. Sgarbossa EB, Pinski SL, Barbagelata A, et al. Electrocardiographic diagnosis of evolving acute myocardial infarction in the presence of left bundle-branch block. N Engl J Med 1996;334:481-487.
26. Kontos MC, McQueen RH, Jesse RL, et al. Can myocardial infarction be rapidly identified in emergency department patients who have left bundle-branch block? Ann Emerg Med 2001;37:431-438.
27. Tabas JA, Rodriguez RM, Seligman HK, et al. Electrocardiographic criteria for detecting acute MI in patients with left bundle branch block: a meta-analysis. Ann Emerg Med 2008;52:329-336.
28. Newby KH, Pisano O, Krucoff MW, et al. Incidence and clinical relevance of the occurrence of bundle-branch block in patients treated with thrombolytic therapy. Circulation 1996;94:2424-2428.
29. Sgarbossa EB, Piniski SL, Gates KB, et al. Early electrocardiographic diagnosis of acute myocardial infarction in the presence of ventricular paced rhythm. Am J Cardiol 1996;77:423-424.
30. Barold SS, Herweg B, Curtis AB. Electrocardiographic diagnosis of myocardial infarction and ischemia during cardiac pacing. Cardiol Clin 2006;24:387-399.
31. Brady WJ, Perron AD, Martin ML, et al. Cause of ST segment abnormality in ED chest pain patients. Am J Emerg Med 2001;19:25-28.
32. Bradley EH, Herrin J, Wang Y, et al. Door-to-drug and door-to-balloon times: Where can we improve? Time to reperfusion therapy in patients with ST segment elevation myocardial infarction (STEMI). Am Heart J 2006;151:1281-1287.
33. Sharkey SW, Berger CR, Brunette DD, et al. Impact of the electrocardiogram on the delivery of thrombolytic therapy for acute myocardial infarction. Am J Cardiol 1994;15:550-553.
34. Gu, YL, Syilaas T, van der Horst IC, et al. Conditions mimicking acute ST-segment elevation myocardial infarction in patients referred for primary percutaneous coronary intervention. Neth Heart J 2008;16: 325-331.
35. Larson DM, Menssen KM, Sharkey SW, et al. "False-positive" cardiac catheterization laboratory activation among patients with suspected ST-segment elevation myocardial infarction. JAMA 2007;298:2754-2760.
36. Klatsky AL, Oehm R, Cooper RA, et al. The early repolarization normal variant electrocardiogram: correlates and consequences. Am J Med 2003;115:171-177.
37. Mehta MC, Jain AC. Early repolarization on scalar electrocardiogram. Am J Med Sci 1995;309:305-311.
38. Wu J, Stork TL, Perron AD, et al. The athlete's electrocardiogram. Am J Emerg Med 2006;24:77-86.
39. Brady WJ. Benign early repolarization: electrocardiographic manifestations and differentiation from other ST segment elevation syndromes. Am J Emerg Med 1998;16: 692-696.
40. Brady WJ, Lentz B, Barlotta K, et al. ECG patterns confounding the ECG diagnosis of acute coronary syndromes: Left bundle branch block, right ventricular paced rhythms, and left ventricular hypertrophy. Emerg Med Clin North Am 2005;23: 999-1025.
41. Aufderheide TP, Brady WJ. Electrocardiography in the patient with myocardial ischemia or infarction. In: Gibler WB, Aufderheide TP, eds. Emergency Cardiac Care, 1st ed. St. Louis: Mosby; 1994: p. 169-216.
42. Larsen GC, Griffith JL, Beshansky JR, et al. Electrocardiographic left ventricular hypertrophy in patients with suspected acute cardiac ischemia: its influence on diagnosis, treatment and short-term prognosis. J Gen Intern Med 1994;9:666-673.
43. Brady WJ, Harrigan RA, Chan T. Acute coronary syndromes. In: Marx, JA ed. Rosen's Emergency Medicine: Concepts and Clinical Practice, 6th ed. St. Louis: Mosby; 2006: 1154-1195.
44. Brady WJ, Ferguson JD, Ullman EA, et al. Myocarditis: emergency department recognition and management. Emerg Med Clin North Am 2004;865-885.
45. Mason JW, O'Connell JB, Herskowitz A, et al. A clinical trial of immunosuppressive therapy for myocarditis. N Engl J Med 1995;333:269-275.
46. Imazio M, Demichelis B, Cecchi E. Cardiac troponin I in acute pericarditis. J Am Coll Cardiol 2003;42:2144-2148.
47. Patel MR, Meine RJ, Lindblad L, et al. Cardiac tamponade in the fibrinolytic era: Analysis of > 100,000 patients with ST-segment elevation myocardial infarction. Am Heart J 2006;151:316-322.
48. Spodick DH. Acute cardiac tamponade. N Engl J Med 2003;349:684-690.
49. Dorfman TA, Agel R. Regional pericarditis: A review of the pericardial manifestations of acute myocardial infarction. Clin Cardiol 2009;32:115-120.
50. Nisbet BC, Breyer M. Acute myopericarditis with focal ECG findings mimicking acute myocardial infarction. J Emerg Med 2008;32:115-120.
51. Tibrewala AV, Asch F, Shah S, et al. Association of size of myocardial scar and persistence of ST-segment elevation after healing of anterior wall myocardial infarction. Am J Cardiol 2007;99:1106-1108.
52. Galiuto L, Barchetta S, Paladini et al. Functional and structural correlates of persistent ST elevation after acute myocardial infarction successfully treated by percutaneous coronary intervention. Heart 2007;93:1376-1380.
53. Antman EM, Braunwald E. ST-elevation myocardial infarction: Pathology, pathophysiology and clinical features. In: Libby P, Bonow RO, Mann DL, et al, eds. Braunwald's Heard Disease: A Textbook of Cardiovascular Medicine, 8th ed. Philadelphia, PA: Elsevier; 2008: 1207-1230.
54. Smith S, Nolan M. Ratio of T amplitude to QRS amplitude best distinguishes acute anterior MI from anterior left ventricular aneurysm. Acad Emerg Med 2003;10:516.
55. Kopelnik A, Zaroff JG. Neurocardiogenic injury in neurovascular disorders. Crit Care Clin 2006;22:733-752.
56. Davis TP, Alexander J, Lesch M. Electrocardiographic changes associated with acute cerebrovascular disease: A clinical review. Prog Cardiovasc Dis 1993;36: 245-260.
57. Cantanzaro JN, Meraj PM, Zheng S, et al. Electrocardiographic T-wave changes underlying cardiac and cerebral events. Am J Emerg Med 2008;26:716-720.
58. van der Blit IAC, Hasan D, Vandertop WP, et al. Impact of cardiac complications on outcome after aneurismal subarachnoid hemorrhage: A meta-analysis. Neurology 2009;72:635-642.
59. Brugada P, Brugada J. Right bundle branch block, persistent STE and sudden cardiac death: A distinct clinical and electrocardiographic syndrome. J Am Coll Cardiol 1992;20:1391-1396.
60. Gussak I, Antzelevitch C, Bjerregaard P, et al. The Brugada syndrome: Clinical electrophysioloic and genetic aspects. J Am Coll Cardiol 1999;33:5-15.
61. Bebarta VS, Summers S. Brugada electrocardiographic pattern induced by cocaine toxicity. Ann Emerg Med 2007;49:827-829.
62. Unlu M; Bengi F; Amasyali B, et al. Brugada-like electrocardiographic changes induced by fever. Emerg Med J 2007;24:e4.
63. Alings M, Wilde A. "Brugada" syndrome clinical data and suggested pathophysiological mechanism. Circulation 1999;99: 666-673.
64 Mattu A, Rogers RL, Kim H, et al. The Brugada syndrome. Am J Emerg Med 2003;21:146-51.
65. Clarkson TB, Prichard RW, Morgan TM, et al. Remodeling of coronary arteries in human and nonhuman primates. JAMA 1994;271:289-294.
Does the electrocardiograph in your emergency department include a computer-generated interpretation on every electrocardiogram printed? Like me, do you often find them less than helpful? Occasionally troubling?Subscribe Now for Access
You have reached your article limit for the month. We hope you found our articles both enjoyable and insightful. For information on new subscriptions, product trials, alternative billing arrangements or group and site discounts please call 800-688-2421. We look forward to having you as a long-term member of the Relias Media community.
