Mechanical Ventilation in the Emergency Department
Mechanical Ventilation in the Emergency Department
Authors: Howard Friedland, DO, FACEP, FACOEP, Newark Beth Israel Medical Center, Osteopathic Program Director, Department of Emergency Medicine, Newark, NJ; and Dana Emery, MD, PhD, Department of Emergency Medicine, Newark Beth Israel Medical Center, Newark, NJ.
Peer Reviewers: Peter M.C. DeBlieux, MD, LSUHSC Director of Faculty and Resident Development, LSUHSC Professor of Clinical Medicine, Tulane University Professor of Clinical Surgery, New Orleans, LA; and Mohamed Y. Rady, MD, PhD, FRCS (Eng.), FRCP (U.K.), FCCM, Professor, Critical Care Medicine, Mayo Clinic College of Medicine, Consultant, Department of Critical Care Medicine, Mayo Clinic Hospital, Mayo Clinic Arizona.
Endotracheal intubation is one of the core procedures for emergency medicine. Traditionally patients who require mechanical ventilation were rushed to the intensive care units often before they were placed on a ventilator. However with the current hospital crowding problem, such patients may spend a large portion of their intensive respiratory care time in the ED. Today's emergency physicians may find themselves responsible for initiating, sustaining, and even weaning patients on mechanical ventilation. This review will assist the emergency physician in providing quality respiratory support to these critically ill patients.
Mechanical ventilation is a life-saving therapy that has become the mainstay of management for patients with acute respiratory failure. The objectives of this review will be to understand indications for mechanical ventilation, to understand the anatomy and physiology of how positive pressure ventilation helps reduce the work of breathing and restore adequate gas exchange, and to understand the basics of both non-invasive positive pressure ventilation (NIPPV) and invasive positive pressure ventilation (IPPV), including lung-protective strategies intended to reduce iatrogenic injury from mechanical ventilation. Common problems and complications will also be identified and addressed.
Since the widespread use of mechanical ventilation began in Scandinavia and the United States in the mid-1950s for the treatment of respiratory distress secondary to paralysis from poliomyelitis, the understanding of the mechanisms of gas exchange, pulmonary mechanics, and heart-lung interactions in relation to mechanical ventilation has increased tremendously. So, too, has the understanding of the complications of mechanical ventilation, which consequently has resulted in changes in recommendations regarding the way in which ventilation is delivered.
Respiratory complaints are present in 12% of emergency department (ED) visits in the United States. Asthma rates are increasing, and patients for whom the ED is their primary source of medical care often may present late during asthma exacerbations. It is thus the responsibility of every emergency physician to be familiar with management techniques involving mechanical ventilation.
—Sandra M. Schneider, MD, FACEP, Editor
Basic Pulmonary Physiology
Understanding basic pulmonary physiology is essential to understanding how to initiate mechanical ventilation; it ensures that the method of gas delivery meshes with the patient' s underlying physiology to avoid ventilator-induced lung injury.
A review of minute volume and alveolar ventilation:
Minute volume: VE = VT x f, where VT = tidal volume and f = frequency (breaths per minute). The amount of air that moves in and out of a patient' s lungs per minute is termed the minute volume (VE).
Tidal volume: VT = VA + VD, where VA = alveolar volume and VD = dead space volume. Normal anatomic dead space volume is calculated as 2.2 mL/kg of ideal body weight, and consists of the volume of the trachea and larger airways in a healthy person. In disease states, in addition to the anatomic dead space, there may be a variable amount of additional "pathologic" dead space consisting of bronchioles and alveoli that are not perfused. Calculation of alveolar minute volume accounts for dead space volume by subtracting it from tidal volume:
Alveolar minute volume: VA = (VT – VD) x f
The alveolar minute volume and the rate of CO2 production by the body determine the partial pressure of CO2 in alveoli (PACO2). Partial pressure of CO2 in systemic arterial blood (PaCO2) is accepted as being approximately the same as partial pressure of CO2 in alveoli (PACO2):
PaCO2 ~ PACO2 = k x (VCO2 / VA)
In which k = 0.863 (at 37°C at normal atmospheric pressure when saturated with water vapor), and where VCO2 = rate of CO2 production by the body (in mL/min) and VA = alveolar minute ventilation (in L/min).
A clinician must keep in mind the effects of a chosen mode of mechanical ventilation as they relate to the patient' s underlying disease state. Parameters to consider (covered in further detail below) include: peak airway pressure, mean airway pressure, tidal volume, frequency of delivered breaths, underlying lung compliance, and inspiratory/expiratory ratio.
Airway pressures during mechanical ventilation:
| Paw | = airway pressure |
| Paw-peak | = peak airway pressure |
| Paw-mean | = mean airway pressure |
| TI | = length of time over which inspiration occurs |
Peak airway pressure (Paw-peak) occurs during inspiration, and is measured in cm H2O. A Paw-peak greater than 35 cm H2O leads to alveolar over-distention and injury. In a pressure-volume curve, increases in pressure without concomitant increase in volume should thus be avoided.
Mean airway pressure (Paw-mean) corresponds to the area under the curve in a pressure over time curve, and correlates with achieved oxygen delivery. The longer the length of time over which inspiration occurs (TI), the greater the Paw-mean, and thus the higher the oxygen delivery per ventilation. In a pressure-volume curve, the area under the curve should thus be maximized. A challenge for the clinician is to avoid excessive Paw-mean while continuing to deliver adequate oxygen delivery per ventilation.
Indications for Mechanical Ventilation
Respiratory failure is a unifying characteristic of conditions that benefit from mechanical ventilation. In the patient with hypercarbic respiratory acidosis, both non-invasive positive pressure ventilation (NIPPV) and invasive positive pressure ventilation (IPPV) have utility in reducing the patient' s work of breathing and thus preventing respiratory muscle fatigue, or alternatively, speeding recovery of respiratory muscles when fatigue is already present. In the patient with hypoxic respiratory failure, both NIPPV and IPPV can help correct hypoxemia by allowing delivery of a high inspired oxygen fraction (FiO2) and reduction of shunting by maintaining flooded or collapsed alveoli open. In a patient with an unprotected or unstable airway (as may accompany a decreased level of consciousness), intubation followed by IPPV allows securing of the airway, reduction of risk of aspiration, and ability to maintain adequate alveolar ventilation.
Respiratory failure can be identified by several definitions:
- PaO2 < 55-60 on maximal FiO2. This is hypoxemic respiratory failure.
- PaCO2 > 50 and pH < 7.30. This is hypercapnic respiratory failure.
- Vital capacity < 10 mL/kg
- FEV1 < 10 mL/kg
- Maximum inspiratory force < 25 cm H2O.
Examples of hypoxemic respiratory failure include exacerbations of chronic obstructive pulmonary disease (COPD) or asthma, pneumonia, pulmonary edema, pneumothorax, pulmonary embolism, and acute respiratory distress syndrome (ARDS). Likewise, hypercapnic respiratory failure may also be found with COPD, severe asthma, pulmonary edema, ARDS, as well as in cases of drug overdose, poisoning, neuromuscular disease, or central nervous system (CNS) injury.
Indications for invasive mechanical ventilation also may be divided into emergent, urgent, delayed, and elective.1 Emergent intubation is indicated for patients that require immediate airway protection and mechanical ventilation on arrival. Urgent intubation is performed for patients with impending airway loss, increased work of breathing with worsening hypoxia and increasing hypercarbia, and/or injuries that may compromise the airway or chest. Delayed intubation is appropriate when a patient is stable enough for primary and secondary assessment, but subsequently requires mechanical ventilation due to progression of disease, inadequate improvement with treatment, or need for transport to a non-critical-care area (for instance, to undergo CT scanning).2 Elective intubation and mechanical ventilation may also prove necessary for airway protection and control during invasive procedures.
Modes of Ventilation
Modes of ventilation may be divided into non-invasive positive pressure ventilation (NIPPV) and invasive positive pressure ventilation (IPPV). NIPPV modes do not utilize an invasive artificial airway; rather, NIPPV requires an awake, alert patient who is cooperative and has an intact respiratory drive and airway. In contrast, IPPV modes utilize an invasive artificial airway such as an endotracheal tube, a nasotracheal tube, or a tracheostomy tube. Both NIPPV and IPPV strategies are discussed in detail below.
Non-Invasive Positive Pressure Ventilation (NIPPV). Over the past several years, non-invasive ventilatory strategies such as continuous positive airway pressure (CPAP) and biphasic positive airway pressure (BIPAP) have come to offer an alternative to traditional invasive ventilation, especially during early presentation of certain respiratory complaints, with greatest benefit noted for patients with COPD, acute pulmonary edema, and immunocompromise.3 Non-invasive ventilation requires an awake, alert patient who is cooperative, has an intact respiratory drive, and has a patent airway. Non-invasive modes of ventilation can provide a temporary bridge during which the patient may improve with therapy and possibly avoid intubation and invasive PPV.
Continuous Positive Airway Pressure (CPAP). CPAP is a useful NIPPV modality for treatment of hypoxemic respiratory failure; by increasing compliance, the patient' s work of breathing is decreased. The use of CPAP requires a tight-fitting mask (either nasal or facial). Starting with a pressure of 0-15 cm H2O, and increasing as tolerated to decrease FiO2 is a recommended strategy.
Biphasic Positive Airway Pressure (BIPAP). BIPAP is a combination of CPAP and positive support ventilation; its use is beneficial in the fatigued patient because BIPAP allows greater support during inspiration. Inspiration is pressure limited and can be triggered either by inspiratory effort or by a timed cycle. The use of BIPAP requires a tight-fitting facemask. Inspiratory positive airway pressure (IPAP) is usually set between 8-20 cm H2O, and expiratory positive airway pressure (EPAP) between 0-15 cm H2O. For this therapy to be effective, there must be a pressure gradient of at least 5 cm between IPAP and EPAP. IPAP should be increased gradually, as tolerated, up to 20 cm H2O, to alleviate dyspnea, decrease respiratory rate, increase tidal volume, and establish patient-ventilator synchrony.
Benefits of NIPPV include improved alveolar ventilation, and decreased work of breathing, mortality, morbidity, length of hospital stay, and need for invasive mechanical ventilation.3 Similar to noninvasive pressure support ventilation (an adjunct used with some modes of IPPV to provide positive airway pressure during inspiration), noninvasive positive airway pressure during expiration can decrease the work of breathing by partially overcoming intrinsic positive end-expiratory pressure; the result is that less patient effort is required to trigger ventilator-assisted breaths. The mortality benefit conferred by NIPPV is likely due in part to a decrease in the incidence of nosocomial infections, especially pneumonia and sepsis.4 The risks of NIPPV include possible barotrauma, pressure necrosis from an ill-fitting mask, and gastric dilatation; NIPPV does not protect against aspiration, and there is no access to the airway for suctioning.
Systematic reviews and meta-analyses of randomized trials of NIPPV have demonstrated its efficacy in treatment of COPD exacerbations.5 A consensus statement from the American Association for Respiratory Care endorses the early use of NIPPV when two or more of the following are present and no contraindications exist:6
- Respiratory distress with moderate to severe dyspnea;
- Arterial pH less than 7.35 with PaCO2 above 45 mmHg;
- Respiratory rate of 25/minute or greater.
In comparison, a systematic review of randomized trials of NIPPV in acute severe asthma exacerbations was less conclusive regarding benefits of NIPPV in this situation: the single included trial on 30 patients showed benefit with NIPPV when compared to usual medical care alone, with significant improvement in hospitalization rate, number of patients discharged from the ED, percent predicted forced expiratory volume in one minute (FEV1), forced vital capacity (FVC), peak expiratory flow rate (PEFR), and respiratory rate.7 These promising preliminary results have yet to be replicated in larger, randomized controlled trials.
Evidence of the beneficial effects of NIPPV in patients presenting with acute respiratory distress syndrome (ARDS), consisting of bilateral pulmonary infiltrates, impaired oxygenation (with PaO2/FiO2 < 200), and absence of elevated left pulmonary artery pressure or elevated pulmonary capillary wedge pressure) has recently been expanded. In a recent multi-center survey, NIPPV improved gas exchange and avoided intubation in 54% of patients presenting with ARDS, and avoidance of intubation was consequently associated with less ventilator-associated pneumonia and a lower intensive care unit mortality rate.8
Contraindications to NIPPV include cardiac or respiratory arrest, non-respiratory organ failure, severe encephalopathy (GCS < 10), severe upper gastrointestinal bleeding, hemodynamic instability or unstable cardiac arrhythmia, facial surgery or trauma or deformity, upper airway obstruction, inability to cooperate/protect the airway, inability to clear respiratory secretions, and high risk for aspiration.9 Respiratory acidosis is not a contraindication to NIPPV. In a case-control study of 64 patients with COPD and severe hypercapnic respiratory failure (mean pH 7.18) who received NIPPV, 38% never required endotracheal intubation, and those who failed NIPPV and required intubation were not harmed by the delayed intubation and prolonged acidemia.10
Both CPAP and BIPAP require careful patient observation; not all patients willingly tolerate these therapies, and of those who do, not all will improve using NIPPV modalities. Some will subsequently require intubation and IPPV. Improvements in pH and PCO2 occurring within two hours predict the eventual success of NIPPV. If stabilization or improvement has not been achieved during this period, the patient should be considered an NIPPV failure and intubation must be considered strongly. Other criteria for a failed NIPPV trial include: worsened encephalopathy or agitation, inability to clear secretions, inability to tolerate any available mask, hemodynamic instability, and worsened oxygenation.
Invasive Positive Pressure Ventilation (IPPV). For patients in respiratory failure who require emergent, urgent, delayed, or elective intubation (including patients who fail a trial of NIPPV), a variety of modes exist for the delivery of oxygen via invasive mechanical ventilation. Each of these includes three key variables: the triggering event that begins inspiration, the parameter that limits air flow, and the method of cycling that ends inspiration. In volume-cycled ventilator modes, the ventilator seeks to deliver a constant preset tidal volume; the parameter that ends inspiration is completion of delivery of that preset VT. Purely volume-cycled ventilatory modes do not, in general, take into account lung compliance. Conversely, in pressure-cycled ventilator modes, the ventilator alters gas flow to achieve a preset airway pressure over a preset inspiratory time (TI), and the targeted airway pressure is achieved with variable gas flow. The ventilator will not exceed the preset pressure limit; as a consequence, variable volume is delivered. The advantage of a pressure-cycled ventilation strategy is that it reduces alveolar over-distention and thus decreases ventilator-induced lung injury.11,12
Pressure and volume targeted ventilation obey the same principles. In pressure-targeted (pressure-controlled) ventilation, an airway pressure target and inspiratory time are preset, while flow and tidal volume become the dependent variables. In volume targeted (volume controlled) ventilation, a target volume and flow (or inspiratory time in certain ventilators) are preset, while pressure and inspiratory time (or flow in the ventilator where inspiratory time is preset) become the dependent variables. Types of invasive mechanical ventilation strategies include those that are primarily volume-controlled, those that are primarily pressure-controlled, and those that offer a combination of pressure and volume control. Common ventilator modes include:
- Controlled Mechanical Ventilation (CMV)
- Assist Control (AC)
- Intermittent Mechanical Ventilation (IMV) (now rarely used)
- Synchronized IMV (SIMV)
- Pressure Regulated Volume Control (PRVC)
- Continuous Positive Airway Pressure (CPAP)
- Pressure Support Ventilation (PSV).
Controlled mechanical ventilation (CMV) should be used only for apneic or paralyzed patients. The ventilator provides breaths at a set rate, regardless of patient effort. The ventilator is set to deliver either a set VT or a set pressure. This mode is rarely used anymore.
In assist control (AC) ventilation, the ventilator delivers preset tidal volume at a set minimum rate. If the patient attempts to draw a breath, the ventilator detects the attempt as a negative pressure below its sensitivity setting; it then delivers a full breath at the preset volume/pressure. Sensitivity may be pressure- or flow-triggered. Since the tidal volume is preset, the patient will receive that volume through a demand valve; the patient cannot reduce the tidal volume. AC is a common initial setting in ventilatory management of intubated patients. The only work of breathing occurs if a patient breathes above the preset respiratory rate, in which case all breaths will be patient initiated. If the patient makes inspiratory efforts at a rate below the preset respiratory rate, these breaths will be assisted and interspersed with machine-initiated breaths. A drawback that must be observed with caution with AC is that if the patient is tachypneic, the minute volume delivered can become too high. AC also covers a host of new modes such as pressure control (PC) or volume control (VC).
In intermittent mandatory ventilation (IMV), which now rarely is used, the ventilator provides breaths at a preset, time-based rate. The patient can initiate spontaneous breaths, but unlike AC, receives only a tidal volume proportional to the time and depth of the patient-initiated inspiration. The patient receives no support for non-mandatory breaths; the tidal volume is completely controlled by the patient. Mandatory breaths are delivered at the preset volume/pressure setting of the ventilator.
With synchronized intermittent mandatory ventilation (SIMV), mandatory breaths are delivered at the preset volume/pressure setting of the ventilator, but the ventilator also coordinates spontaneous and machine breaths. Pressure support is also available for the patient' s spontaneous breaths. The effect of this synchronization is to prevent delivery of a scheduled breath on top of a spontaneous breath or during exhalation. If the patient makes a spontaneous inspiratory effort during the next machine-delivered breath, the delivered breath is synchronized with the patient' s effort. If the patient makes an inspiratory effort not in synchronization with the preset machine breaths, the patient receives an unassisted breath determined by effort.
In pressure regulated volume control (PRVC), a guaranteed tidal volume is delivered using a "pressure control" waveform, meaning that the pressure target is adjusted to the least value that satisfies the targeted tidal volume minimum. In this mode, the minimum VE, the minimum frequency, and the inspiratory time per cycle are all preset. An advantage of PRVC is that the ventilator automatically adjusts to compliance changes in the patient' s lungs; it dynamically titrates the pressure needed to reach to the preset tidal volume via a decelerating inspiratory flow pattern. This characteristic of PRVC permits a guaranteed tidal volume, yet limits trauma from excessive volumes and prevents hypoventilation. A disadvantage of PRVC is that the pressure delivered is dependent on the tidal volume achieved on the previous breath; if the patient intermittently makes a significant respiratory effort, this can result in very variable tidal volumes with both high and low volumes being delivered.
Continuous positive airway pressure (CPAP) is used only for spontaneously breathing patients, and is covered in further detail above as a mode of NIPPV. CPAP may be used for non-invasive ventilation or for weaning from invasive ventilation. Continuous positive airway pressure reduces the effort of inhalation, and holds airways open during exhalation.
Pressure support ventilation (PSV) is an adjunct used with SIMV or with CPAP, and also only in spontaneously breathing patients. The pressure support is given on the patient-initiated breaths. Gas flow accompanies each inspiration to overcome the resistance of the ventilator circuit and reach the desired VT. The usual starting setting is 10 cm H2O. Support stops when the flow rate reaches 25% of maximum. Variants of pressure support, such as volume support, also exist.
Choosing Initial Ventilator Settings in IPPV
Ventilator settings that the clinician will need to select for a patient requiring IPPV include mode, rate, FiO2, volume vs. pressure control, initial VT, and initial pressure. Additionally, waveform, flow, inspiration to expiration (I:E) ratio, sensitivity to patient-triggered breaths, and positive end-expiratory pressure (PEEP) may be modified to optimize treatment in accordance with the patient' s underlying pathology.
Mode of Ventilation. The initial mode of ventilation will most often be either AC (PRVC in some centers) or SIMV; AC is the most common initial mode of ventilation. The preset rate of breath delivery (in the patient without special considerations such as severe asthma or ARDS) is most often 10-12 breaths/minute. The initial FiO2 is usually 95-100%, followed by attempts to wean to 60% to reduce oxygen toxicity. The choice of volume controlled vs. pressure-controlled ventilation may depend upon the patient' s underlying lung compliance.
Tidal Volume. Initial tidal volume for a patient without underlying lung disease is usually calculated at approximately 8-10 mL/kg of ideal body weight. In patients with severe asthma or ARDS, lung-protective ventilatory strategies have evolved that utilize a smaller VT of 6-8 mL/kg of ideal body weight, higher frequency, and permissive hypercapnea (covered further below). If using pressure support ventilation in conjunction with IMV/SIMV, the usual starting value is 10 cm H2O and may be titrated up to 30 cm H2O.
Ventilator Waveform. In selecting the ventilator waveform for optimal ventilation of a patient, consider the differences between a square waveform and a decelerating (ramp) waveform. In a square waveform, once the maximum inspiratory flow rate is achieved, a constant flow rate continues until the preset volume is delivered. The advantage of a square waveform is that it allows for a longer time of expiration (TE), and is thus better suited for patients with COPD or brain injury who can benefit from a longer TE. In contrast, in a decelerating (ramp) waveform, once the maximum inspiratory flow rate is achieved, the rate of gas delivery slows; the result is a longer inspiratory time (TI), lower peak airway pressures (Paw-peak), and higher mean airway pressures (Paw-mean). In summary, the decelerating waveform can provide higher oxygenation with less pulmonary trauma in a patient who does not require a longer TE.
Ventilator Flow Rate. In selecting the ventilator flow rate (the rate of gas delivery in L/min), a customary starting value is 60 L/min, and may range from 10-160 L/min. The advantage of a faster flow rate is that it permits a longer passive exhalation time; however, this must be tempered with the possibility of a decreased inspiratory time leading to hypoxia. Caution must be used if increasing flow rate, as increased flow leads to turbulent flow in the airways, causing increased airway pressures.
Inspiration:Expiration Ratio. In normal spontaneous breathing, the inspiration:expiration (I:E) ratio is approximately 1:4. In intubated patients, the I:E ratio is usually 1:2 to 1:4. A ratio with more inspiration time may improve oxygenation, but can lead to air trapping, elevated intrapulmonary pressures, and CO2 retention. In patients with outflow-restrictive conditions such as COPD or severe asthma, an I:E ratio with greater time allowed for expiration time is therefore beneficial. If the ventilator does not allow direct setting of TI, the I:E ratio may be indirectly set either by lowering the respiratory rate per minute or by increasing the flow rate; since TI equals tidal volume divided by flow rate, increasing the flow rate decreases TI and consequently increases TE.
Sensitivity. The sensitivity of the ventilator is the negative pressure required to trigger delivery; this value is usually set at 1-2 cm H2O. If this ventilator setting is too sensitive, an adverse consequence is that the ventilator may auto-cycle causing an artificial tachypnea. The patient may become tachypneic from unintended triggering. Conversely, if this ventilator setting is too insensitive, a high work of breathing will be required from the patient to trigger delivery of a breath. Flow-triggered breaths are available on many modern ventilators. The mechanism for triggering a breath is a decrease in the background flow generated by the ventilator. This may be set as liters per minute, or percent decrease, depending upon the ventilator manufacturer.
Positive End Expiratory Pressure (PEEP). Positive end expiratory pressure (PEEP) may be generated extrinsically by the ventilator (PEEPE), or may arise intrinsically when exhalation is incomplete (PEEPI). Intrinsic PEEP increases in the setting of airway obstruction, insufficient exhalation time, and consequent air trapping, and may cause the ventilator to be less able to sense respiratory effort by the patient. The benefit of either physiological or extrinsically generated PEEP is that it helps keep the large non-cartilaginously supported airways and the smaller alveoli open in order to prevent collapse, atelectasis, and hypoxia at the end of expiration. This triad of collapse, atelectasis, and hypoxia worsens lung compliance and is associated with ventilator-induced lung injury. PEEP can increase functional residual capacity and improve oxygenation; however, PEEP does not increase CO2 clearance. Initial PEEP ventilator settings range from 3-20 cm H2O4; it is customary to start with 5 cm H2O (sometimes described as replicating "physiological" PEEP), and increase PEEPE until hypoxia improves, with the accompanying goal of weaning to FiO2 less than 60%.
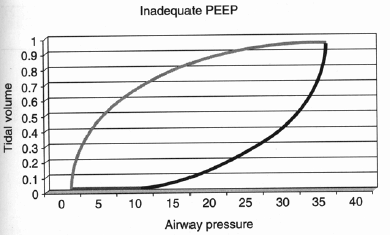
Another way to evaluate whether adequate PEEP is present is to observe the pressure–volume loops generated by the ventilator. In Figure 1,1 the lower arrow denotes inspiration while the upper arrow indicates exhalation. Note that as soon as there is delivered pressure to the airway, there is an increase in measured tidal volume. This indicates that the airways are open and do not need to be forced open by increasing the pressure in the airway; "optimal PEEP" has been achieved. In contrast, the pressure-volume loop in Figure 21 indicates inadequate PEEP. Compare the curve in Figure 2 to that in Figure 1: Note that the lower segment of this loop (denoting inspiration) is initially flat along the X-axis, indicating that there is inadequate PEEP to keep smaller airways open. Once the airway pressure is high enough to open the alveolar units, then each increase in airway pressure is matched by a corresponding increase in tidal volume.
| Figure 1. Dynamic Pressure-Volume Loop |
|
| The lower arrow denotes inspiration while the upper arrow indicated exhalation. Note that as soon as there is delivered pressure to the airway, there is an increase in measured tidal volume. This indicates that the airways are open and do not need to be forced open by increasing the pressure in the airway. If this latter case were true (see Figure 2), then the P-V loop would initially be flat along the X-axis. (Used with permission from Roberts JR, Hedges JR, eds. Clinical Procedures in Emergency Medicine. 4th edition. Philadelphia: Elsevier Inc.; 2004: 146-170.) |
| Figure 2. Inadequate PEEP and the Pressure-Volume Loop |
|
| Compare this curve to that in Figure 1: Note that the lower segment of this loop (denoting inspiration) is initially flat along the X-axis, indicating that there is inadequate PEEP to keep smaller airways open. Once the airway pressure is high enough to open the alveolar units, then each increase in airway pressure is matched by a corresponding increase in tidal volume. (Used with permission Roberts JR, Hedges JR, eds. Clinical Procedures in Emergency Medicine. 4th edition. Philadelphia: Elsevier Inc.;2004:146-170.) |
Be cautious regarding side effects of PEEP. Increased PEEP can cause hemodynamic compromise via increased intrathoracic pressure, decreased venous return, and thus decreased cardiac output.14 Desired levels of PEEP may not be possible because of deleterious effects of cardiac compression and collapse. Since volume depletion compounds this problem, it is essential that a patient be adequately volume resuscitated. Please see Insert for suggested settings for common conditions requiring mechanical ventilation.
Complications Associated with IPPV
Complications associated with invasive mechanical ventilation may result from the endotracheal tube or tracheostomy15; alternatively, ventilator-associated pneumonia,16,17 oxygen toxicity,18 or mechanical lung injury may occur.19-21 Specific complications from ventilator malfunction and operator error include inappropriate ventilator settings, alarm failure, accidental disconnection from the ventilator, inadequate humidification of inspired air, and over or under heating of inspired air. Detrimental effects of PPV can include decreased cardiac output secondary to increased intrathoracic pressure and decreased venous return,22,23 as well as trauma to the lungs themselves. It is essential to adequately sedate any patient undergoing mechanical ventilation in order to avoid agitation.
Types of Lung Injuries Resulting from IPPV. Barotrauma, which has been suggested to occur in 10-20% of patients on mechanical ventilation,29 refers to gross air leaks that are due to the development of an excessive pressure difference between an alveolus and its adjacent bronchovascular sheath.25,26 Barotrauma is often due to overdistention from high PEEPE or PEEPI (as mentioned above), rather than to high Paw-peak, and includes sequelae such as pneumothorax, pneumomediastinum, subcutaneous emphysema, and pneumoperitoneum. Pneumoperitoneum has been observed in 4% of patients receiving high PEEP.27 The detrimental consequences of mechanical ventilation on cardiac output are more pronounced in the setting of concurrent hypovolemia and/or decreased vascular tone (as seen, for instance, with sepsis or neurogenic shock).
Volutrauma refers to a more subtle type of lung injury that can occur secondary to pulmonary over-distention induced by mechanical ventilation; the critical variable for injury is not the airway pressure itself, but rather the volume that results in excessive end-inspiratory lung stretch.28-32 Excessive inspiratory lung stretch can lead to development of pulmonary edema, diffuse alveolar damage, and increased epithelial and microvascular permeability.33-35 Pressure-volume loops are also useful in identifying alveolar overdistention. In Figure 3,1 increases in airway pressure without accompanying increases in measured tidal volume lead to a plateau of the P-V curve, known as the "bird's beak" profile. This profile is a reasonable indicator of alveolar overdistension and airway injury.
| Figure 3. Alveolar Overdistention |
|
| Alveolar overdistention is reflected in this pressure-volume curve in which there is an increase in airway pressure at the end of inspiration without any concomitant increase in tidal volume. This pressure-volume curve pattern approximates a "bird's beak" profile, which is a reasonable indicator of airway injury. (Used with permission from Roberts JR, Hedges JR, eds. Clinical Procedures in Emergency Medicine. 4th edition. Philadelphia: Elsevier Inc.; 2004: 146-170.) |
Atelectrauma refers to the damage that can occur when lungs are allowed to become atalectatic and then are re-expanded. Collapse and re-expansion of alveoli has been shown to be a critical factor causing injury.31,36 Ventilator (or volume)-induced lung injury (VILI) involves damage to lung parenchyma. High inflation pressures place patients at risk for VILI; patients especially at risk include those with poor lung compliance who require higher pressures to ventilate (for example, patients with pulmonary edema, obstructive pulmonary disease, or ARDS). Overdistention of alveoli causes injury and release of cytokines, chemokines, and activation of endothelial factors, which combine to cause an inflammatory cascade called biotrauma.19,37
While the additional inflammatory response and lung injury that results from mechanical ventilation of injured lungs leads to an increase in lung cytokines, under conditions in which there is increased lung permeability, these cytokines may translocate from the alveolar space to the systemic circulation.38 Cytokines produced during lung injury include tumor necrosis factor (TNF), a key mediator in the systemic inflammatory response syndrome that may play a role in multi-organ failure. Release of cytokines and/or inflammatory mediators into the systemic circulation may be a mechanism by which mechanical ventilation might effect systemic consequences and lead to the development of end-organ failure.39,40 In a randomized controlled trial in ARDS patients ventilated with either a conventional ventilatory strategy (tidal volume of 10-12 mL/kg, PEEP set at lowest value to maintain adequate oxygenation) or a protective strategy designed to minimize lung stress, there was a significant decrease in lavage and serum cytokines in the ventilator group treated with the protective strategy.41 The concept of biotrauma may be the missing link that explains why most patients who die with ARDS succumb not because their lungs fail, but because of the development of multiple organ dysfunction syndrome.39,42
Lung-Protective Ventilation in ARDS. How can ventilator-induced lung injury be reduced? The ARDS (Acute Respiratory Distress Syndrome) Network compared a more conventional VT of 12 mL/kg with a plateau airway pressure limit of 50 cm H20 with a "lung protective" strategy of a VT of 6 mL/kg and a plateau airway pressure limit of 30 cm H2O, with the option to use a VT as low as 4 mL/kg and give NaHCO3 for acidosis. In the ARDS Network study, the lung-protective strategy resulted in a 22% reduction in mortality and 20% fewer days requiring ventilation, and the study was stopped early to extend these benefits.42 The current lung-protective ventilatory strategy in treatment of patients with ARDS incorporates a low VT, an increased respiratory rate, limiting the plateau airway pressure to 30 cm H2O, permissive hypercapnea, PEEP, and decreased I:E ratio.
The use of PEEP in patients with ARDS can help achieve adequate hemoglobin saturation and decrease the requirement for a high FiO2. In this setting, the application of PEEP results in an increase in functional residual capacity (also referred to as "recruitment" of alveoli), which decreases intrapulmonary shunting of blood through regions with collapsed alveoli and improves ventilation/perfusion matching.43 The degree of PEEP-induced alveolar recruitment further correlates with improvement in PaO2.44 Although applied PEEP is beneficial to oxygenation, a higher PEEP strategy has not yet been shown to confer additional reduction in mortality when ventilation with low tidal volumes (6 mL/kg ideal body weight) is used in unselected ARDS patients.45
Lung-Protective Ventilation in Obstructive Airway Disease. Patients with obstructive airway disease experience air trapping and hyperinflation of lungs, which leads to both elevated peak and elevated plateau airway pressures, and places these patients at high risk for VILI. The current improved ventilatory strategy employed in treating such patients also incorporates a low VT, but is different than the strategy used for ARDS in that patients with obstructive airway disease require a low respiratory rate and high flow rate to maximally increase their expiratory time.46,47 This strategy of permissive hypercapnea/controlled hypoventilation with resultant acidosis is thought to be safer than experiencing the high airway pressures noted during conventional ventilation of patients with obstructive airway disease.48 Controlled hypoventilation becomes even more important for an intubated asthmatic patient than a patient with COPD, as asthmatic patients who require intubation already have relatively high long-term mortality rates.49,50 Also essential for continued treatment of this type of respiratory failure are continued in-line administration of bronchodilators via a large diameter oral endotracheal tube,51 and adequate sedation and paralysis, as agitation/discomfort from ventilation on awake patients can lead to tachypnea, overbreathing, and dyssynchrony with the ventilator.
Permissive Hypercapnea Strategy. Acute hypercapnia can lead to severe acidemia and neurological dysfunction. However, when carbon dioxide levels are allowed to increase gradually, the resulting acidosis is less severe because of renal buffering, and the elevation in arterial PCO2 is generally well tolerated. The use of supplemental oxygen and PEEP during a permissive hypercapnic ventilation strategy allows for substantial reduction of minute ventilation without jeopardizing oxygenation.52 Furthermore, hypercapnic acidosis shifts the oxyhemoglobin curve to the right, promoting oxygen release at the tissue level. Negative systemic effects of permissive hypercapnic ventilation strategies include cerebral vascular dilatation (which can increase ICP), and lowering of the seizure threshold. Therefore, permissive hypercapnic ventilation is considered to be contraindicated in patients with cerebrovascular disease and in those with a seizure disorder because of its effects on cerebrovascular tone and seizure threshold.52
In evaluation of permissive hypercapnea as a strategy for invasive ventilatory management of obstructive airway disease, it was found that although patients tolerate a pH as low as 7.25, a pH less than 7.2 requires treatment.52,53 Treatment may be attempted by increasing the respiratory rate. If this is not feasible, treat with NaHCO3 (150 mEq/L of IV fluid).
Troubleshooting
Effective IPPV requires a number of related systems to all be functioning properly. In troubleshooting the patient on IPPV, it is important to systematically review these systems to determine the cause of an apparent malfunction. The first system is the delivery of utilities to the ventilator itself. This includes electricity for the ventilator, oxygen, and possibly air to drive the ventilator. The next system is the ventilator itself. After the ventilator is the tubing circuit connecting the ventilator to the patient. The next step is the artificial airway allowing access to the patient' s trachea. This is usually an endotracheal tube, but could be a tracheostomy tube as well. The final step is the patients' airway itself, staring from the trachea to mainstem bronchus, left and right bronchi and then to smaller airways.
Each breath requires oxygen to flow from the wall outlet through tubing to the ventilator. The ventilator then will deliver the appropriate volume or pressure over time through the circuit to the endotracheal tube, and ultimately in the patient' s trachea and then smaller airways. Malfunctions can occur at any step along the way, and may include a problem in more than one system.
The initial evaluation and management of a ventilated patient who appears to be in respiratory distress should include a rapid assessment of appearance, vital signs (including pulse oximetry), cardiac rhythm, the patency of the ventilator circuit connections, and the position and condition of the artificial airway, including a check for air leaking from the nose or mouth. If these actions do not identify the cause of patient distress, a chest x-ray, arterial blood gas, and electrocardiogram should be obtained. A chest x-ray is central to the evaluation of respiratory distress in ventilated patients; important findings include the position of the endotracheal tube as well as signs of pneumonia, pulmonary edema, pneumothorax, pleural effusion, or atelectasis. A tension pneumothorax may be treated presumptively prior to obtaining a chest x-ray by needle decompression followed by placement of a thoracostomy tube.
When confronted with the patient with sudden respiratory distress or change in status, the issue may be a ventilator problem or a patient-centered problem. If the issue is a ventilator problem, the patient' s ventilatory status will likely be improved by removal from the ventilator and manual ventilation with 100% oxygen while improper settings and/or leaks are sought and corrected. Any patients who appear unstable should be removed from the ventilator and bagged with 100% oxygen.
If the issue is a patient-centered problem, respiratory distress in mechanically ventilated patients can be classified anatomically as originating in the airway, the pulmonary parenchyma, or outside the lungs. Common causes include endotracheal tube obstruction, heart failure, pneumonia, patient-ventilator asynchrony due to inadequate sedation, and compression of the lungs by pleural fluid or ascites.
Problems related to the endotracheal tube include obstruction (by secretions, blood, kinking, a foreign body, or by the patient biting upon the tube), migration of the ETT, and ETT cuff malfunction. In-line suctioning, administration of bronchodilators, and repositioning and/or replacement of the ETT can alleviate many of these issues. Problems related to the pulmonary parenchyma and vasculature include atelectasis, pneumonia, aspiration of oropharyngeal or gastric contents, pulmonary edema, and pulmonary emboli; these conditions can all cause respiratory distress in patients undergoing mechanical ventilation. With the exception of acute pulmonary embolism, these conditions can usually be differentiated by physical examination and plain chest radiography. Pulmonary embolism is difficult to diagnose. Although CT scans and angiograms are diagnostic, they require transportation that may be difficult in severely ill patients.
If a ventilator system continues to alarm and the patient does not appear to be receiving its intended benefit, take the patient off the ventilator and use manual bag-valve-ETT ventilation. Bagging the patient allows you to feel the compliance of their lungs. A patient that was easy to bag earlier but now is difficult indicates the problem is with either the airway or intrinsic to the patient. If the patient bags easily and oxygenates well, there is a problem with the ventilator and associated tubing, or with the ventilator settings. The ventilator and tubing should be checked. Any problems found should be corrected. If the system is functioning properly, the ventilator settings should be adjusted or the patient may require additional sedation to improve synchrony with the ventilator.
If the patient is difficult to bag, evaluate the patient for pneumothorax. Consider needle thoracostomy if unilaterally decreased breath sounds are noted. Another possibility for difficulty ventilating is high levels of auto-PEEP. This can be improved by disconnecting the patient' s endotracheal tube and allowing a prolonged exhalation. If the patient is easy to bag after a pause, resume ventilation and adjust the ventilator.
In the stable patient, examine the patient and the ventilatory system. Has the ETT become dislodged? Has there been an accidental disconnection within the system? Is the oxygen attached and on? If the patient becomes hypotensive during ventilation, consider causes and corrective actions. Is the hypotension due to decreased preload, to dynamic hyperinflation, to tension pneumothorax, to pulmonary embolism?
Problems outside the lungs can cause respiratory distress by restricting inspiration or increasing respiratory drive. Thoracic processes that cause restriction include pneumothorax and large pleural effusions. Abdominal problems, such as ascites, ileus, or splinting from abdominal pain, may limit chest wall expansion and lower the compliance of the respiratory system.54 Fever, shock, pain, delirium, and anxiety can all increase respiratory drive and cause apparent respiratory distress and agitation without a change in respiratory mechanics or gas exchange. These are diagnoses of exclusion, however, and life-threatening disorders must always be considered before administering sedatives and analgesics.
Proximal airway pressure waveforms can be useful in identifying the cause of an acute deterioration in respiratory status in a patient receiving IPPV.55 If the peak pressure is increased but the plateau pressure is unchanged, the problem is an increase in airway resistance: consider the presence of obstruction of the tracheal tube or smaller airways by secretions or bronchospasm. If the peak and plateau pressures are both increased, the problem is a decrease in distensibility of the lungs and chest wall: consider the possibility of pneumothorax, lobar atelectasis, acute pulmonary edema, worsening pneumonia or ARDS, increased abdominal pressure, or development of auto-PEEP. If the peak pressure is decreased, the problem many be a systemic air leak: consider manually ventilating the patient and listening for a cuff leak. A flow diagram in Figure 4 shows how changes in peak and plateau pressures can provide a qualitative assessment of lung mechanics at the bedside of a patient receiving IPPV.
Lower than expected airway pressures would indicate a likely leak in the system, allowing air to escape. This could be a true leak in the tubing, or a cap off of one of the ports on the tubing. Significant leaking around the endotracheal tube can also cause this. In general the patient' s inspired and expired minute volumes should be equal in a closed system, such as a ventilator. Unequal volumes indicate a leak in the system. One common benign cause of unequal volumes is the use of nebulized bronchodilators. Since the nebulizer is attached to the tubing, the extra volume is noted on the expired minute volume. But, since the volume was not delivered by the ventilator itself, it is not noted on the inspired volume reported on the ventilator. This will cause a high expired minute volume alarm on most ventilators, but not indicate a true malfunction in the system.
Conclusions
An emergency physician will see and treat many patients with acute respiratory failure; not all of these will require intubation initially, but emergency physicians must be prepared to manage mechanical ventilation of any patient in respiratory distress who does not improve. NIPPV has become the first-line treatment in an attempt to reverse acute respiratory failure in patient populations with COPD, acute pulmonary edema, or immunocompromise. For patients who do undergo intubation and IPPV, whether performed emergently, urgently, delayed, or electively, lung-protective strategies should be incorporated that reduce risk of iatrogenic injury to the patient' s lungs from IPPV; namely, lower tidal volumes, judicious tailoring of respiratory rate to the patient' s underlying pulmonary condition, careful monitoring to avoid damaging PEEP levels, and permissive hypercapnia.
References
1. Roberts JR, Hedges JR, eds. Clinical Procedures in Emergency Medicine. 4th edition. Philadelphia: Elsevier Inc.; 2004: 146-170.
2. Venkataranam ST, Orr RA. Intra-hospital transport of critically ill patients. Crit Care Clin 1992; 8:525-531.
3. Liesching T, Kwok H, Hill NS. Acute applications of noninvasive positive pressure ventilation. Chest 2003;124:699-713.
4. Girou E, Brun-Buisson C, Taille S, et al. Secular trends in nosocomial infections and mortality associated with noninvasive ventilation in patients with exacerbation of COPD and pulmonary edema. JAMA 2003;290:2985-2991.
5. Ram FS, Picot J, Lightowler J, Wedzicha JA. Non-invasive positive pressure ventilation for treatment of respiratory failure due to exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2004; CD004104.
6. Bach JR, Brougher P, Hess DR, et al. Consensus Conference IV Noninvasive positive pressure ventilation. Respir Care 1997;42:364-449.
7. Ram FS, Wellington S, Rowe BH, et al. Non-invasive positive pressure ventilation for treatment of respiratory failure due to severe acute exacerbations of asthma. Cochrane Database Syst Rev 2005; CD004360.
8. Antonelli M, Conti G, Esquinas A, et al. A multiple-center survey on the use in clinical practice of noninvasive ventilation as a first-line intervention for acute respiratory distress syndrome. Crit Care Med 2007;35:388-390.
9. International Consensus Conference In Intensive Care Medicine. Noninvasive positive pressure ventilation in acute respiratory failure. Am J Respir Crit Care Med 2001;163:283-291.
10. Squadrone E, Frigerio P, Fogliati C, et al. Noninvasive vs invasive ventilation in COPD patients with severe acute respiratory failure deemed to required ventilatory assistance. Intensive Care Med 2004;30:1303-1310.
11. Wang SH, Wei TS. The outcome of early pressure-controlled inverse ratio ventilation on patients with severe respiratory distress syndrome in surgical intensive care unit. Am J Surg 2002;183:151-155.
12. Pinhu L, Whitehead T, Evans T, et al. Ventilator-associated lung injury. Lancet 2003;361:332-340.
13. Broussarsar M, Thierry G Jaber S, et al. Relationship between ventilatory settings and barotrauma in the acute respiratory distress syndrome. Intensive Care Med 2002;28:406-413.
14. Kirby RR, Perry JC, Calderwood HW, et al. Cardiorespiratory effects of high positive end-expiratory pressure. Anesthesiology 1975;43:533-539.
15. Kollef MH, Ahrens TS, Shannon W. Clinical predictors and outcomes for patients requiring tracheostomy in the intensive care unit. Crit Care Med 1999;27:1714-1720.
16. Cunnion KM, Weber DJ, Broadhead WE, et al. Risk factors for nosocomial pneumonia: Comparing adult crucial-care populations. Am J Respir Crit Care Med 1996;153:158-162.
17. American Thoracic Society: Hospital acquired pneumonia in adults: Diagnosis, assessment of severity, initial antimicrobial therapy, and preventative strategies. Am J Respir Crit Care Med 1996;153:1711-1725.
18. Thiel M, Chouker A, Ohta A, et al. Oxygenation inhibits the physiological tissue-protecting mechanism and thereby exacerbates acute inflammatory lung injury. PLoS Biology 2005; 3:e174.
19. Tremblay NJ, Slutsky AS. Ventilator-induced injury: from barotraumas to biotrauma. Proc Assoc Am Physicians 1998;110:482-488.
20. Plotz FB, Slutsky AS, Van Vught AJ, et al. Ventilator-induced lung injury and multiple system organ failure: a critical review of facts and hypotheses. Intensive Care Med 2004;30:1865-1872.
21. Lionetti V, Recchia FA, Ranieri MV. Overview of ventilator-induced lung injury mechanisms. Curr Opin Crit Care 2005;11:82-86.
22. Tittley JG, Fremes SE, Weisel RD et al. Hemodynamic and myocardial metabolic consequences of PEEP. Chest 1985;88:496-502.
23. Biondi JW, Schulman DS, Soufer R, et al. The effect of incremental positive end-expiratory pressure on right ventricular hemodynamics and ejection fraction. Anesthesia and Analgesia 1988;67:144-151.
24. Gammon RB, Shin MS, Buchalter SE. Pulmonary barotraumas in mechanical ventilation. Patterns and risk factors. Chest 1992;102:568-572.
25. Rouby JJ, Lherm T, Martin De Lassale E, et al. Histologic aspects of pulmonary barotraumas in critically ill patients with acute respiratory failure. Intensive Care Med 1993;19:383-389.
26. Pierson DJ. Alveolar rupture during mechanical ventilation: Role of PEEP, peak airway pressure, and distending volume. Respir Care 1988:33:472.
27. Beilin B, Shulman DL, Weiss AT, et al. Pneumoperitoneum as the presenting sign of pulmonary barotraumas during artificial ventilation. Intensive Care Med 1986;12:49-51.
28. Dreyfuss D, Saumon G. Barotrauma is volutrauma, but which volume is the one responsible? Intensive Care Med 1992;18:139-141.
29. Dreyfuss D, Saumon G. Role of tidal volume, FRC, and end-inspiratory volume in the development of pulmonary edema following mechanical ventilation. Am Rev Respir Dis 1993;148:1194-1203.
30. Dreyfuss D, Saumon G. Ventilator-induced lung injury: Lessons from experimental studies. Am J Respir Crit Care Med 1998;157:294-323.
31. Slutsky AS. Lung injury caused by mechanical ventilation. Chest 1999;116:9S-15S.
32. Vlahakis NE, Hubmayr RD. Cellular stress failure in ventilator-injured lungs. Am J Respir Crit Care Med 2005;171:1328-1342.
33. Webb HH, Tierney DF. Experimental pulmonary edema due to intermittent positive pressure ventilation with high inflation pressures. Protection by positive end-expiratory pressure. Am Rev Respir Dis 1974;110:556-565.
34. Parker JC, Hernandez LA, Longenecker GL, et al. Lung edema caused by high peak inspiratory pressures in dogs. Role of increased microvascular filtration pressure and permeability. Am Rev Respir Dis 1990;142:321-328.
35. Parker JC, Hernandez LA, Peevy KJ. Mechanisms of ventilator-induced lung injury. Crit Care Med 1993;21:131-143.
36. Chu EK, Whitehead T, Slutsky AS. Effects of cyclic opening and closing at low- and high-volume ventilation on bronchoalveolar lavage cytokines. Crit Care Med 2004;32:168-174.
37. Belperio JA, Keane MP, Lynch JP 3rd, et al. The role of cytokines during the pathogenesis of ventilator-associated and ventilator-induced lung injury. Semin Respir Crit Care Med 2006;27:350-364.
38. Tutor JD, Mason CM, Dobard E, et al. Loss of compartmentalization of alveolar tumor necrosis factor after lung injury. Am J Respir Crit Care Med 1994;149:1107-1111.
39. Slutsky AS, Tremblay LN. Multiple system organ failure. Is mechanical ventilation a contributing factor? Am J Respir Crit Care Med 1998;157:1721-1725.
40. Borrelli E, Roux-Lombard P, Grau GE, et al. Plasma concentrations of cytokines, their soluble receptors, and antioxidant vitamins can predict the development of multiple organ failure in patients at risk. Crit Care Med 1996;24:392-397.
41. Ranieri VM, Suter PM, Totorella C, et al. Effect of mechanical ventilation on inflammatory mediators in patients with acute respiratory distress syndrome. JAMA 1999;282:54-61.
42. The Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 2000;342:1301-1308.
43. Gattinoni L, Pelosi P, Crotti S, Valenza F. Effects of positive end-expiratory pressure on regional distribution of tidal volume and recruitment in adult respiratory distress syndrome. Am J Respir Crit Care Med 1995;151:1807-1814.
44. Malbouisson LM, Muller JC, Constantin JM, et al. Computed tomography assessment of positive end-expiratory pressure-induced alveolar recruitment in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med 2001;163:1444-1450.
45. Brower RB, Lanken PN, MacIntyre N, et al. Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med 2004;351:327-336.
46. Connors AF Jr, McCaffree DR, Gray BA. Effect of inspiratory flow rate on gas exchange during mechanical ventilation. Am Rev Respir Dis 1981;124:537-543.
47. Laghi F, Segal J, Choe WK, et al. Effect of imposed inflation time on respiratory frequency and hyperinflation in patient with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2001;163:1365-1370.
48. Tuxen DV. Permissive hypercapnic ventilation. Am J Respir Crit Care Med 1994;150:870-874
49. Darioli R, Perret C. Mechanical controlled hypoventilation in status asthmaticus. Am Rev Respir Dis 1984;129:385-387.
50. Marquette CH, Saulnier F, Leroy O, et al. Long-term prognosis of near-fatal asthma. A 6-year follow-up study of 145 asthmatic patients who underwent mechanical ventilation for a near-fatal attack of asthma. Am Rev Respir Dis 1992;146:76-81.
51. Rodrigo G, Rodrigo C. Assessment of the patient with acute asthma in the emergency department: A factor analytic study. Chest 1993;104:1325-1328.
52. Feihl F, Perret C. Permissive hypercapnia: How permissive should we be? Am J Respir Crit Care Med 1994;150:1722-1737.
53. Bidani A, Tzouanakis AE, Cardenas VJ, et al. Permissive hypercapnia in acute respiratory failure. JAMA 1994;272:957-962.
54. Gilroy RJ Jr, Lavietes MH, Loring SH, et al. Respiratory mechanical effects of abdominal distension. J Appl Physiol 1985;58:1997-2003.
55. Marino PL. The ICU Book. 2nd edition. Baltimore: Lippincott Williams & Wilkins; 1997: 428-430.
Today's emergency physicians may find themselves responsible for initiating, sustaining, and even weaning patients on mechanical ventilation. This review will assist the emergency physician in providing quality respiratory support to these critically ill patients.Subscribe Now for Access
You have reached your article limit for the month. We hope you found our articles both enjoyable and insightful. For information on new subscriptions, product trials, alternative billing arrangements or group and site discounts please call 800-688-2421. We look forward to having you as a long-term member of the Relias Media community.