Probing further: Ultrasound evaluation of pneumothoraces
Probing further: Ultrasound evaluation of pneumothoraces
Author: Teresa S. Wu, MD, Director of Simulation Education and Training, Attending Staff Physician, Department of Emergency Medicine, Orlando Regional Medical Center, Orlando, Florida
Peer Reviewer: Eric Legome, MD, Assistant Professor of Emergency Medicine; Director, New York University/ Bellevue Emergency Medicine Residency, New York University School of Medicine, New York
Introduction
Using bedside ultrasound to detect pneumothoraces was first introduced in a veterinary journal in 1987. In 1989, Wernecke and colleagues published the first report on the ability of ultrasound to diagnose pneumothoraces in humans. Because air and bone are considered traditional ultrasound obstacles, the benefits of pulmonary ultrasonography were not fully recognized until just recently.
As practice evolved and applications expanded, surgeons and emergency physicians began utilizing bedside ultrasound for detection of pneumothorax as part of the trauma patient evaluation. Ultrasound's portability, easy learning curve, and ability to provide quick and noninvasive information, has inspired physicians in multiple specialties to incorporate pulmonary scanning into their daily practice.
In a normal lung, the parietal and visceral pleura glide against each other during respiration. On ultrasound, this gliding can be clearly visualized and has been termed 'lung sliding'. Apposition of the parietal and visceral pleura in normal lung produces hyperechoic streaks farfield to the pleural line called 'comet tail' artifacts. (See Figure 1.) The presence of air in between the parietal and visceral pleura inhibits lung sliding and the production of comet tail artifacts. Absence of lung sliding and comet tail artifacts has been shown to support the diagnosis of a pneumothorax in many recent studies. This article provides a comprehensive summary of the most current data concerning the use of ultrasound to detect pneumothoraces, and outlines the manner by which it can be used to augment your clinical practice. Table 1 highlights the studies included in this article.
| Figure 1. Ultrasound of Normal Lung with a Linear Transducer |
|
| Figure 1. Ultrasound of Normal Lung with a Linear Transducer. P-V: parietal-visceral pleural interface where horizontal lung sliding is noted in real time imaging. C: vertical comet tail artifact extending across the entire image of the parenchyma. Rev: horizontal reverberation echo. |
Table 1. Summary of Studies Evaluating Ultrasound Detection of Pneumothoraces |
|
How sensitive and specific is it?
Source: Knudtson JL, et al. Surgeon-performed ultrasound for pneumothorax in the trauma suite. J Trauma 2004; Mar;56(3):527-30.
Dr. Knudtson and colleagues prospectively enrolled 326 trauma patients during a 7-month study period in an attempt to analyze the ability of ultrasound to detect pneumothoraces. Patients were excluded if the trauma center did not have properly trained staff members available to perform an ultrasound evaluation at the time of their arrival, if the patients did not undergo a chest radiograph for comparison, and if the patients' hemodynamic instability precluded an ultrasound evaluation. A pneumothorax was diagnosed by ultrasound if the patient had absence of lung-sliding and comet tail artifact. Absence of lung-sliding was observed in 313 subjects, with 312 of these subjects having a confirmed pneumothorax on subsequent chest radiographs. The one false-negative ultrasound finding was from a very small apical pneumothorax that was managed conservatively. Twelve patients had a pneumothorax that was diagnosed on both ultrasound study and on chest radiograph. One patient with subcutaneous emphysema on presentation had false-positive findings on ultrasound. The authors concluded that although ultrasound should not be used as a substitute for chest radiography, bedside pulmonary ultrasonography can be used as a valuable adjunct in the early diagnosis and management of patients suspected of having a pneumothorax.
Commentary
Pneumothoraces should be easy to detect in clinical scenarios where a thin, healthy patient presents to a quiet room for evaluation, and a thorough history and physical examination can be performed without distractions. For most trauma patients, this is not the typical means by which they are evaluated and treated. Dr. Knudtson's large prospective investigation highlights the high sensitivity (92.3%) and specificity (99.6%) of bedside ultrasound in detecting a pneumothorax in a busy Level 1 trauma center. Interestingly, the data from this study were obtained using a curvilinear ultrasound probe. In previous studies, high frequency linear transducers were utilized for their ability to produce enhanced images. Because initial pulmonary scanning studies had been performed primarily with the linear probe, suggestions to extend the focused assessment with sonography for trauma (FAST) examination to include a lung scan were complicated by the need to change probes in the midst of the evaluation. If larger studies can show comparability in using the curvilinear footprint versus the linear probe, the prospect of expanding the FAST examination to include pulmonary imaging may be easier than expected.
Emergency departments (EDs) utilize chest x-rays as an initial screening tool for pneumothoraces in trauma patients, however, chest x-rays are not 100% sensitive or specific for a pneumothorax. Comparing the results of bedside ultrasound with a chest radiograph and a follow-up computerized tomography (CT) scan of the chest would have made this a more useful study. It is clear from subsequent studies that chest radiographs should not be used as the gold standard for comparison because they miss a significant number of pneumothoraces.
Beyond just detection
Source: Blaivas M, et al. A prospective comparison of supine chest radiography and bedside ultrasound for the diagnosis of traumatic pneumothorax. Acad Emerg Med 2005;Sep;12(9):844-9.
Dr. Blaivas and colleagues compared the accuracy of ultrasound versus chest radiography in detecting a pneumothorax and evaluated whether ultrasound can be used to delineate the size of a pneumothorax. Chest CT, or the release of air on chest tube placement, was used as the gold standard. In their study, 176 eligible patients underwent ultrasonographic lung evaluation by emergency physicians in 4 defined views: 2nd anterior intercostal space at the midclavicular line, 4th intercostal space at the anterior axillary line, 6th intercostal space at the midaxillary line, and 6th intercostal space at the posterior axillary line. During evaluation of the parietal and visceral pleura interface, power Doppler was applied over the pleural interface if lung sliding was not easily detected. The presence of 'power slide' indicated a normal lung evaluation. Study physicians were also asked to categorize all detected pneumothoraces into small (≤ 10%), medium (11%-40%), and large (≥ 40%). When lung sliding was not visible in the 2nd anterior midclavicular intercostal space or at the 4th intercostal space at the anterior axillary line, the pneumothorax was considered small. When it was absent from views at the 6th intercostal space of the midaxillary line, the pneumothorax was deemed medium. Large pneumothoraces were thought to be present when lung sliding was not visible at the 6th intercostal space of the posterior axillary line. Portable, supine anterioposterior (AP) chest radiographs were performed on all eligible patients shortly after ultrasonographic evaluation. Chest CT scans were obtained at the discretion of the treating physician.
A pneumothorax was detected in 53 patients by ultrasound, and in 40 patients on chest radiography. Of the 53 patients with a positive ultrasound, 52 were found to have a corresponding pneumothorax on CT scan. One patient with a large right-sided lung contusion seen on CT scan had been reported to have a false-positive pneumothorax diagnosed on ultrasound. One false-negative ultrasound was noted in a patient who had a ≤1% pneumothorax discovered on CT. This patient was managed conservatively. In this study, ultrasound findings were not affected by the presence of subcutaneous emphysema in 3 patients. There were 23 large, 11 medium, and 19 small pneumothoraces detected on CT. Although there was good correlation between CT and ultrasound approximation of pneumothorax size in small and large pneumothoraces, discrepancies were noted with medium-sized pneumothoraces. Despite a small sample size, the authors concluded that ultrasound is more sensitive than a portable supine AP chest radiograph in the diagnosis of traumatic pneumothoraces.
Commentary
This was one of the largest studies dedicated to evaluating the utility of ultrasound detection of pneumothoraces by emergency medicine physicians. Furthermore, of the studies available, few have looked at multiple pulmonary views in the evaluation of traumatic pneumothoraces. This paper introduced 4 practical views of the parietal-visceral pleural interface that can help physicians detect and quantify pneumothoraces. The novel application of Power Doppler to enhance visualization of parenchymal and pleural movement can augment clinical judgment when sonographic interference is present, or when resolution is limited. As mentioned earlier, most of the data available on ultrasound detection of pneumothoraces have been obtained using linear transducers. Application of Power Doppler (Figure 2) to scans performed with the curvilinear probe may help balance the inferior picture quality noted with a curved array of crystals. Utilizing the 4 views introduced in this paper also will help physicians to roughly quantify pneumothorax size. For example, in a patient undergoing serial examinations for potential progression of a known pneumothorax, ultrasound approximations of pneumothorax size may potentially save the patient from additional radiation from chest x-rays or a CT scan. Although the technique is not perfect at discerning the difference between a large and medium pneumothorax, taken in conjunction with the clinical picture, a rough estimate may help guide management options.
| Figure 2. Ultrasound Image of Normal Lung with Color Doppler Applied |
|
| Figure 2. Ultrasound Image of Normal Lung with Color Doppler Applied. P-V: parietal-visceral interface where horizontal lung sliding is observed in real time imaging. Rev: horizontal reverberation echo. CD: Color Doppler applied at the prietal-visceral interface to highlight normal lung sliding (AKA Power slide). |
Searching for the 'silent' ones
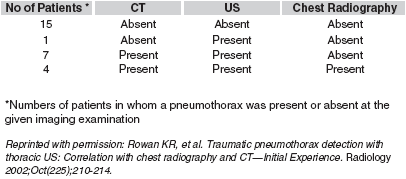
Source: Rowan KR, et al. Traumatic pneumothorax detection with thoracic US: Correlation with chest radiography and CT—Initial Experience. Radiology 2002;Oct(225);210-214.
In this study, Dr. Rowan and colleagues prospectively compared the accuracy of ultrasound versus supine chest radiography in the detection of traumatic pneumothoraces. During the 8-month study, 70 trauma patients suspected of having a pneumothorax were enrolled. Twenty-seven of these patients underwent a thoracic CT study based on clinical guidelines, and their results were used in the study (Table 2).
| Table 2. Pneumothorax Depiction at CT, US, and Chest Radiography |
|
All patients underwent an ultrasound evaluation in a supine position by either a staff radiologist or a radiology resident within minutes of their chest radiograph. Patients were diagnosed with a pneumothorax if lung sliding and comet tail artifacts were absent at the pleural interface. A CT scan of the chest was used as the reference standard for diagnosis verification. Ultrasound was found to be superior to chest radiography in detecting a pneumothorax, with a sensitivity of 100% and a specificity of 94%. Ultrasound findings and the chest radiograph agreed in 15 patients without a pneumothorax, and in 4 patients with a pneumothorax. Seven patients had a pneumothorax that was not detected on chest radiography, but was found on ultrasound evaluation. One patient was incorrectly thought to have a pneumothorax on ultrasound, but not one on chest radiograph. A subsequent CT scan showed the presence of a large anterior bulla and substantial bullous emphysema.
Because patients with large pneumothoraces requiring needle or tube thoracostomy were excluded from this study, the authors concluded that ultrasound is better than a supine chest x-ray in detecting small, 'silent' pneumothoraces.
Commentary
Although studies have compared ultrasound's ability to detect a pneumothorax with the diagnostic accuracy of supine chest radiography, this was one of the first to use a CT scan of the chest to confirm the diagnoses. The results indicated that ultrasound is as good, if not better, than chest radiography in detecting a clinically occult pneumothorax. Large, symptomatic pneumothoraces would be treated immediately by needle or tube thoracostomy, and neither a chest x-ray nor an ultrasound evaluation would necessarily be required prior to intervention and treatment. In situations where a chest x-ray is not readily obtainable, or intubation is imminent with positive-pressure ventilation, performing a bedside ultrasound of the lungs can be a quick, noninvasive way to validate an initial clinical concern. The inability of ultrasound to distinguish an emphysematous bleb from an acute pneumothorax, and the chance of chronic pulmonary disease producing a false-positive result on ultrasound, are limitations that have surfaced as an increasing number of physicians are taking advantage of this new ultrasound application. The authors recognized that their small sample size was a limitation to the study, and that further studies with larger, diverse patient populations will need to show similar results before this new ultrasound application can be universally accepted.
More than 'lung sliding' & 'comet tails'
Source: Lichtenstein DA, et al. Ultrasound diagnosis of occult pneumothorax. Crit Care Med 2005; Jun;33(6): 1231-8.
Lichtenstein and colleagues performed a retrospective study evaluating 200 consecutive patients during a 10-year period to test the hypothesis that ultrasonographic lung evaluations can decrease the need for chest CT scans. Medical and surgical ICU patients were included in the review if they underwent an ultrasound examination, chest radiograph, and CT scan. Ultrasound was performed at 6 defined thoracic points for evaluation of the following signs:
- Lung sliding: a dynamic sign of the parietal pleura sliding against the visceral pleura in a normal lung.
- A lines: repetition of horizontal artifacts of normal lung parenchyma deep to the pleural line.
- B lines: vertical comet tail artifacts arising from the pleural line, extending to the bottom edge of the screen. These artifacts obstruct A line visualization and move with lung sliding. Note that the presence of an abundance of B lines can indicate interstitial lung disease. The presence of B lines rules out pneumothorax.
- Z lines: vertical comet tail artifacts arising from the pleural line that do not reach the bottom of the screen. These artifacts do not obstruct A line visualization, and are independent of lung sliding. Both normal lung and pneumothorax can exhibit Z lines.
- O lines (non-A/non-B lines): when neither vertical nor horizontal artifacts were observed.
- M-mode lung sliding: in M-mode (motion mode), normal lung sliding below the pleura will produce a homogenous granular pattern, or the 'seashore' sign (Figure 3). With a pneumothorax, horizontal lines replace this granular pattern (Figure 4).
- Lung point: visualization of the edge of the pneumothorax, where lung sliding and absence of lung sliding are seen on the same image.
Figure 3. Ultrasound in M Mode of a Normal Lung |
|
| Figure 3. M-Mode Ultrasound of Normal Lung. P-V: parietal-visceral interface. Note that the movement of normal lung parenchyma farfield to the parietal-visceral interface produces a granulose pattern on M-mode imaging. This is also known as the 'seashore' sign. |
Figure 4. Ultrasound in M Mode of Pneumothorax |
|
| Figure 4. M-mode Ultrasound of a Pneumothorax. PP: parietal pleura. Air has been trapped between the parietal and visceral pleura, preventing propagation of sound waves deep to the parietal pleura. No motion artifact can be transmitted farfield to the parietal pleura. |
The authors excluded 4 cases with emphysema, and 2 cases because dressings prohibited ideal scanning conditions. The final study groups consisted of 43 pneumothorax cases and 302 controls. Thirty-one of the 43 pneumothorax cases were missed using chest radiography, while all 43 cases were correctly diagnosed using ultrasound. Of the 302 controls, lung sliding was present in 237 patients, but absent in 65, yielding a sensitivity of 100% and a specificity of 78%.
The A line sign without the presence of associated B lines was thought to signify a pneumothorax (sensitivity of 95%, specificity of 62%). Combining the presence of A lines, in the absence of B lines and lung sliding, sensitivity remained at 95% but specificity increased to 94%. When using lung point as a sign of occult pneumothorax, ultrasound had a sensitivity of 79% and a sensitivity of 100%. A correlation also was made between the presence of lung point and the need for chest tube insertion, suggesting that large volume pneumothoraces will produce lung point on ultrasound evaluation.
From this, the authors concluded that ultrasound can be effectively used to evaluate for pneumothorax when the physician caring for the patient is the one performing the ultrasound evaluation and is careful to observe for the presence or absence of A lines, B lines, Z lines, M mode findings, lung point, and lung sliding.
Commentary
Most of the literature cites the absence of comet tail artifacts and lung sliding on ultrasound as the primary indication for diagnosing a pneumothorax. In this article, Lichtenstein and colleagues defined multiple other ultrasound findings that must be considered before the diagnosis of a pneumothorax should be made. Both B lines and Z lines are comet tail artifacts that arise from the pleural interface. B lines should extend the full length of the screen, and it is their absence that indicates a pneumothorax is present. However, Z lines are comet tail artifacts that also arise from the pleural interface, but diminish within a few centimeters. Z lines should not be confused with B lines because Z lines can be seen in both normal lungs and those with a pneumothorax.
The authors also made a salient point that an ultrasound diagnosis of pneumothorax should not be limited to the absence of lung sliding alone. In cases of acute respiratory distress syndrome, extensive pneumonia, emphysema, or pleural adhesions, lung sliding may be absent even though a pneumothorax is not present concomitantly. Technical deficiencies, dynamic noise filters, and problems with probe frequency can also prevent visualization of lung sliding in a normal lung. It may take more than the evaluation of lung sliding and standardization of what is known as the comet tail artifact to maximize ultrasound's sensitivity and specificity for detecting a pneumothorax. When multiple sonographic findings are considered, the use of ultrasound as an adjunct in the decision process to diagnose a pneumothorax is quick, noninvasive, and easy to apply to daily practice.
Better than a chest radiograph?
Source: Chung JM, et al. Value of high-resolution ultrasound in detecting a pneumothorax. Eur Radiol 2005;15: 930-5.
The authors of this study evaluated the ability of ultrasound to detect small, acute pneumothoraces in patients who had just undergone transthoracic needle aspiration and biopsy of their lungs. Following the procedure, pulmonary ultrasound images were obtained from the anterior chest wall with the patient lying in a supine position. A portable, supine or semi-erect chest radiograph was taken immediately after ultrasound. Patients also underwent a CT scan of the chest to be used as the reference standard for comparison.
All images were reviewed by 4 certified radiologists blinded to the study. Of the 97 study patients, 35 pneumothorax cases were detected using CT. Using the absence of lung sliding to confirm the presence of a pneumothorax, ultrasound was found to have a sensitivity of 80% and a specificity of 94% when compared with CT. In comparison, chest radiography had a much lower sensitivity of 47% and a specificity of 77%. Significant interobserver variation was found in the chest radiograph interpretations, but not during ultrasound evaluation. The authors concluded that the diagnostic accuracy of ultrasound is superior to that of chest radiography in detecting acute pneumothoraces.
Commentary
It is difficult to perform an ultrasound study whereby participants and observers are blinded to the clinical scenario. The majority of the data available has come from studies where the clinician performing the scan is also responsible for interpreting the images obtained. When a physician obtains an ultrasound study of a patient's lung that has suffered acute penetrating injury, interpretation of the images may be biased toward diagnosing a pneumothorax. Dr. Chung and colleagues did a great job designing a blinded study using ultrasound images stored in a cine-review mode to minimize bias. Because all radiograph, ultrasound, and CT scan images were randomized, radiologists were able to make official reads in the absence of confounding factors. Once again, ultrasound was shown to have superior sensitivity and specificity as compared with chest radiography. Despite such a high sensitivity and specificity, it is important to recognize the limitations of this goal-directed ultrasound application. As noted in this study, false-positive ultrasound findings can be seen in patients with a history of pleural disease. Pulmonary disease processes can interfere with normal lung sliding on ultrasound evaluation. It is important to take note of such limitations when incorporating pulmonary sonographic findings into your decision-making process.
Taking it one step further
Source: Kirkpatrick AW, et al. Hand-held thoracic sonography for detecting post-traumatic pneumothoraces: the extended focused assessment with sonography for trauma (EFAST). J Trauma 2004;Aug;57(2):288-95.
Although most pneumothoraces can be diagnosed clinically or on chest radiograph, many still can be missed on initial evaluation. The high sensitivity and specificity of ultrasound in detecting pneumothoraces has prompted many experts to suggest the incorporation of pulmonary scanning into the initial FAST. In this article, Dr. Kirkpatrick and colleagues prospectively evaluated the use of ultrasound as an extension of the FAST examination, designated as the EFAST. From July 2000 through October 2002, eligible trauma patients underwent pulmonary ultrasound prior to any other thoracic imaging studies. Patients were excluded if they had extensive subcutaneous emphysema or chest drains already in place. Investigators scanned the anterior midclavicular 2nd intercostal space and the midaxillary 4th intercostal space in search of normal lung sliding and comet tail artifacts from the pleural interface. Patient's served as their own internal control by having both thoraces examined.
Of the 225 eligible patients, 65 pneumothoraces were identified by CT scan in 52 patients (13 were bilateral). In 5 bilateral pneumothorax cases, the pneumothorax in the contralateral lung was missed on ultrasound. In 7 bilateral pneumothorax cases, both pneumothoraces were missed. Of the 65 CT-confirmed pneumothoraces, 33 cases were identified with EFAST examination with only 3 false-positive evaluations (58.9% sensitivity and 99.2% specificity). In 6 cases, pneumothoraces were diagnosed with chest x-ray that were not diagnosed on ultrasound. In 16 cases, ultrasound picked up pneumothoraces missed on chest x-ray. Calculated likelihood ratios and positive-predictive value of the EFAST examination compared with chest x-ray and CT scan showed that ultrasound of the lung can be very useful when normal lung sliding and comet tail artifacts are visualized.
Commentary
Most studies examining the utility of ultrasound in detecting pneumothoraces do little more than emphasize the high sensitivity and specificity of ultrasound as compared with chest radiography. The authors of this study took a step further and provided a comprehensive discussion of the limitations and pitfalls associated with ultrasound detection of pneumothorax. Three patients had false-positive ultrasound findings. One had subcutaneous emphysema impeding a clear view of lung sliding. Two had right main-stem endotracheal intubations preventing the left lung from generating normal lung sliding on ultrasound evaluation of the left hemithorax.
The authors also reported that the EFAST examination performed poorly in cases of bilateral pneumothoraces. In the study, 12 of the 13 bilateral pneumothorax cases had either one or both pneumothoraces missed on ultrasound evaluation. The authors believed that this discrepancy may be accredited to the loss of an internal standard for comparison. In most studies on this topic, the scan of the thorax had been limited to one midclavicular anterior 2nd intercostal space view. In this study, researchers rightly acknowledged that limiting the number of thoracic views can lead to missed pneumothoraces. One false-negative finding was reported in their study when an investigator only examined the 2nd intercostal spaces bilaterally. An occult pneumothorax was later identified on CT scan. Acknowledging the pitfalls and recognizing the limitation of thoracic ultrasonography for pneumothoraces is a major step toward the collective acceptance of this goal-directed ultrasound application. Further studies may help clarify the overall positive and negative predictive value and define its clinical role.
Do the small ones really matter?
Source: Tam MM, et al. Occult pneumothorax in trauma patients: should this be sought in the focused assessment with sonography for trauma examination? Emerg Med Australasia 2005;17 (5-6):488-93.
Ultrasound has been touted for its ability to detect occult pneumothoraces missed on chest radiography. Experts have proposed that lung scanning for pneumothorax should be incorporated into the initial FAST examination of trauma patients. In this study, Tam and colleagues attempted to determine the actual incidence of occult pneumothoraces in trauma patients and to define their implications for patient management. In a retrospective chart review of trauma patients evaluated during a 17-month period, 143 trauma patients received a FAST examination (93.7% blunt injuries, 6.3% penetrating injuries). Of these 143 patients, only 3 (2.1%) were found to have an occult pneumothorax found on CT and not on initial chest radiography. Two of these 3 cases of occult pneumothorax required chest drains after admission; the remaining patient was managed conservatively. Of the 143 study patients, 6 did not have any type of imaging performed (chest radiography or CT). No adverse outcomes were reported during their hospitalization. Instead of performing an ultrasound study on the lungs of all trauma patients, the authors recommended scanning only high-risk patients: those who have a history of thoracic and/or abdominal injury plus one or more of the following:
- Injury mechanisms potentially causing major trauma
- Abnormal chest examination finding
- Abnormal chest x-ray finding in the absence of pneumothorax
The authors concluded that the detection of small occult pneumothoraces by ultrasound may not warrant further management and, therefore, may not need to be mandated for all patients as part of the FAST examination.
The majority of the studies that have examined ultrasound's ability to detect pneumothoraces have been geared toward promoting its superior sensitivity and specificity in detecting occult pneumothoraces missed on chest radiography. Few have addressed how these results will actually change our clinical practice. It has been shown in multiple studies that ultrasound is quite accurate in detecting the presence of a pneumothorax, but also, that ultrasound is unreliable in quantifying the size of a pneumothorax.
In 2005, Blaivas and colleagues described an approach to thoracic ultrasound that may aid determination of the size of the pneumothorax.1 Ultrasound confirmation of a large pneumothorax is useful in situations where findings were missed on clinical examination and chest x-ray, and during serial examinations to rule out progression of small pneumothoraces. In these situations, positive ultrasound findings may prompt a physician to insert a chest drain.
But what do you do if you find a small occult pneumothorax on ultrasound? Many fear that these small pneumothoraces should be empirically treated with a chest drain to prevent progression in certain clinical scenarios (e.g., the patient is to undergo positive-pressure ventilation, or long operative interventions for other injuries). In studies by Garramone2 and Wolfman3, small occult pneumothoraces were shown to be safely managed with close observation alone, even if the patient required positive-pressure ventilation.
Although enlightening, it is unclear if the data from these studies should be taken as a hard and fast rule for the management of small pneumothoraces. As proposed in this study, it is reasonable to include a thoracic scan into the FAST examination of all high-risk trauma patients. If an occult pneumothorax is discovered at that time, it is important to reflect on the ultrasound findings in conjunction with the entire clinical scenario before a decision is made to withhold or place a chest drain.
References
1. Blaivas, et al A prospective comparison of supine chest radiography and bedside ultrasound for the diagnosis of traumatic penumothorax. Acad Emerg Med 2005;Sept;12(9):844-9.
2. Garramone RR, et al. An objective to measure and manage occult pneumothorax. Surg Gynecol Obstet 1991 Oct:173(4): 257-61.
3. Wolfman NT, et al. Validity of CT classification on management of occult pneumothorax: a prospective study. AJR Am J Roentgenol 1998 Nov;171(5):1317-20.
Clarify the confusion with chronic lung disease
Source: Simon BC, et al. Two cases where bedside ultrasound was able to distinguish pulmonary bleb from pneumothorax. J Emerg Med 2005; Aug; 29(2):210-5.
In patients with chronic emphysema, it is often very difficult to distinguish a pulmonary bleb from a pneumothorax on chest radiography. The ability to distinguish between the two during a resuscitation attempt may save a patient from receiving an unnecessary needle thoracostomy or chest drain. In this study, 2 cases were described where the use of pulmonary ultrasound aided in the diagnosis of bullous disease versus pneumothorax.
The first patient was an elderly gentleman who presented with tachycardia, tachypnea, increased dyspnea on exertion, and decreased breath sounds appreciated in the right upper lobes. A chest radiograph showed large, septated spaces, without lung markings in the entire right upper lobe. Mediastinal structures were shifted toward the left. Ultrasound of his lungs showed the presence of lung sliding and comet tail artifacts, supporting the diagnosis of giant emphysematous bullae without co-existent pneumothorax.
The second patient was a young gentleman with a history of tuberculosis and chronic lung disease who presented with tachypnea, tachycardia, and hypoxia. Lung examination revealed decreased breath sounds on the right side, and chest radiography showed decreased lung markings throughout both lung fields. Bedside ultrasound revealed the absence of lung sliding and comet tail artifacts, indicating that the patient had an occult pneumothorax not clearly delineated on chest radiograph.
The authors suggested that thoracic ultrasound can be used to supplement chest x-ray findings in distinguishing between emphysematous diseases versus pneumothorax.
Commentary
Performing a bedside thoracic ultrasound is a quick, noninvasive way to provide additional information on a patient in whom a pneumothorax is considered. Recognizing its limitations will enable physicians to avoid making erroneous conclusions based on their ultrasound findings.
A few earlier articles have noted that false-positive results can be found in patients with emphysematous lung disease. If the patient has suffered from pleural fibrosis for whatever reason, the adherent parietal and visceral pleura will not exhibit normal lung sliding on ultrasound evaluation. Although normal lung sliding is not present, the apposition of the pleural interface still should provide normal comet tail artifact if a pneumothorax is not present. Similarly, in a patient with a chest radiograph showing either a pulmonary bleb versus a pneumothorax, thoracic ultrasound may not show typical lung sliding if the bleb abuts the parietal pleura, but comet tail artifact still should be evident if no air has leaked in between the parietal and visceral pleura.
Dr. Simon and colleagues briefly mentioned the use of M-mode (motion mode) to augment sonographic findings seen with normal B-mode scanning. In M-mode, movement of the pulmonary structures in a normal lung will produce a grainy snowstorm-like image deep to the pleural line. With a pneumothorax, the air in between the parietal and visceral pleura will prevent proper propagation of sound waves, leading to the appearance of linear streaking or horizontal lines across the M-mode image. In patients with emphysematous lung disease in whom pneumothorax is suspected, B-mode and M-mode ultrasound can be utilized to help rule out the presence of a pneumothorax if lung sliding, comet tail artifacts, and granular 'snowstorm' images are visualized.
Learning from the limitations
Source: Slater A, et al. COPD can mimic the appearance of pneumothorax on thoracic ultrasound. Chest 2006; 129(3);545-550.
In this article, the authors performed a prospective, blinded study to define the sensitivity and specificity of pneumothorax detection with ultrasound in patients with chronic lung disease. Upright posteroanterior (PA) chest x-rays and ultrasound studies were performed within 30 minutes of each other on 9 patients with a pneumothorax, 9 patients with cystic fibrosis, 17 patients with chronic obstructive pulmonary disease (COPD), and 6 control patients. Sonographic movie clips were obtained from 3 thoracic points: anterior 2nd intercostal space, anterior 4th intercostal space, and 6th intercostal space in the mid-axillary line. A radiology attending physician and a radiology resident were separately asked to interpret the images as either "definitely no pneumothorax," "probably no pneumothorax", "uncertain", "probably a pneumothorax", or "definitely a pneumothorax."
In the control group, all 6 were correctly identified to have no pneumothorax. All 9 cystic fibrosis patients were correctly identified as not having a pneumothorax by the attending radiologist; the resident radiologist called 4 of them "probably no pneumothorax", and 5 of them "definitely no pneumothorax." The attending physician correctly identified the presence of a pneumothorax in all 9 pneumothorax patients; the resident failed to diagnose 2 cases (one was labeled uncertain, the other as definitely not a pneumothorax). In 5 of the COPD patients, the attending physician incorrectly diagnosed a pneumothorax. The resident incorrectly thought that 2 cases probably had a pneumothorax, and was uncertain about 4 cases. The overall sensitivity and specificity were 100% and 84%, respectively for the attending physician, and 78% and 81%, respectively for the resident.
Commentary
The authors of this study confirmed that ultrasound is quite sensitive and specific for the evaluation of pneumothorax. The reported sensitivity of 100% for the attending physician is a bit higher than most of the previous studies, but may be due in large part to their evaluation of 3 sonographic findings (lung sliding, comet tail artifact, and lung point) versus the typical 2 findings of lung sliding and comet tailing described in previous trials. Furthermore, data from this study were obtained from scans of 3 anatomical regions on each individual patient. Evaluation of the entire hemithorax will enable practitioners to detect occult pneumothoraces that may not be clearly visualized if only one region of the chest is evaluated. If time only permits one sonographic view of the chest, the scan should be directed to the least-dependant portion of the thorax because air will tend to rise and collect at that point. Remember that the least-dependant point may be at the lung apices or 2nd intercostal space, and pleural movement may not be as pronounced in this region. Evaluating for multiple sonographic signs, such as lung sliding, comet tail artifact, and lung point, will increase your study's sensitivity and specificity.
In patients with COPD, the authors believed that lung hyperexpansion can cause decreased movement between the parietal and visceral pleura, thereby minimizing the amount of lung sliding visualized on ultrasound. The idea that pleural adhesions can prevent lung sliding was not demonstrated in this study, as all 9 cystic fibrosis patients were correctly identified as not having a pneumothorax. Until larger studies can be performed detailing the different sonographic findings expected with varying pulmonary diseases, ultrasound's high specificity suggests that it should only be used in conjunction with the entire clinical scenario to exclude a pneumothorax. If a patient with chronic lung disease has a scan suggesting the presence of a pneumothorax, remember that the sensitivity of ultrasound in detecting pneumothorax is not high enough to warrant reliance on these findings alone.
Conclusions
The ability of ultrasound to accurately detect or exclude the presence of a pneumothorax offers physicians an attractive, noninvasive adjunct to clinical tools already available. In this goal-directed application, pulmonary ultrasound has been shown to be safe, simple, quick, and easy to master. The advantages of ultrasound are further complemented by reports indicating a sensitivity of 73% to 100%, and specificity near 100% with use of either a linear transducer or a curvilinear probe. Although studies that did not utilize CT scanning as the gold standard for comparison should be interpreted cautiously, as a whole, the data have suggested that ultrasound is equal to — if not better than — chest radiography in the evaluation of a pneumothorax. In situations where an immediate CT scan can not be safely obtained, bedside ultrasound can be utilized to help clarify an equivocal chest x-ray or physical examination finding in a challenging clinical scenario.
Much of the praise for pulmonary ultrasound stems from its ability to detect occult pneumothoraces missed on clinical examination and chest radiography. Further studies are needed to elucidate the risk in allowing these seemingly insignificant pneumothoraces to go undetected and undiagnosed. Most patients who are in respiratory distress from a pneumothorax will have findings suggestive of a pneumothorax on examination or on chest x-ray. The role of ultrasound in these patients is less evident. Where pulmonary ultrasound will prove to be most useful is in patients who present with respiratory distress and have pulmonary blebs or equivocal chest x-ray findings that warrant further investigation prior to intervention. The use of pulmonary ultrasound in these situations may help clarify a complicated clinical presentation and save patients from receiving unnecessary invasive treatments.
As more and more physicians become comfortable utilizing pulmonary ultrasound, limitations to its use will inevitably surface. Thus far, it is unclear how accurate sonographic findings truly are in patients with chronic lung disease. In most of the studies evaluated, the main concern lies in the potential for ultrasound to produce a false-positive result. Until these discrepancies can be clarified, ultrasound should primarily be used to help exclude the presence of a pneumothorax. Because ultrasound is not perfectly sensitive for detecting a pneumothorax in patients with chronic pulmonary disease, caution should be taken in performing invasive treatments based on sonographic findings alone.
Studies to date have suggested that the advantages of pulmonary ultrasound have outweighed many of the limitations that have arisen, but clearly more outcome studies are needed. A comprehensive search for sonographic signs (e.g., lung sliding, comet tail artifacts, lung point, and 'seashore' sign on M mode) may add very useful information to any clinical scenario, in a matter of minutes. Many centers have not only adopted the practice of incorporating pulmonary ultrasound into their evaluation of patients with chest pain, but also have expanded their FAST examinations to include an ultrasound evaluation for pneumothorax in all trauma patients. With its growing popularity and universal acceptance, the use of pulmonary ultrasound to detect pneumothoraces will inevitably reach clinical arenas beyond the prehospital setting, emergency department, trauma suite, and even to space travel.
Using bedside ultrasound to detect pneumothoraces was first introduced in a veterinary journal in 1987. In 1989, Wernecke and colleagues published the first report on the ability of ultrasound to diagnose pneumothoraces in humans.Subscribe Now for Access
You have reached your article limit for the month. We hope you found our articles both enjoyable and insightful. For information on new subscriptions, product trials, alternative billing arrangements or group and site discounts please call 800-688-2421. We look forward to having you as a long-term member of the Relias Media community.