Respiratory Virus-Associated Apnea
Authors: Dante A. Pappano, MD, MPH, Attending Physician, Eastern Tennessee Children's Hospital, Knoxville, TN; Ellen S. Bass, MD, FAAP, MPH, Attending Physician, Eastern Tennessee Children's Hospital, Knoxville, TN
Peer Reviewer: Ghazala Q. Sharieff, MD, FACEP, FAAEM, FAAP, Director of Pediatric Emergency Medicine, Palomar-Pomerado Health System/California Emergency Physicians; Associate Professor, Children's Hospital and Health Center/University of California, San Diego
Apnea is a frightening event for the parent and a challenging diagnostic evaluation for the emergency department (ED) physician. As respiratory syncytial virus (RSV) season invades the ED, it is critical to be aware of pediatric patients at risk for apnea. The authors review the pathophysiology, diagnostic approach, and therapeutic options.
— The Editor
History
The understanding that otherwise mild respiratory illness may be associated with apnea grew out of the mid to late 20th century. It was a consequence of advances in the sudden infant death syndrome (SIDS) literature, the advent of viral isolation techniques, and the focus that was placed on viral respiratory disease due to the antibiotic era reduction of bacteria-related respiratory deaths. Like many medical advances, the knowledge was built slowly by many participants. Nevertheless, there are certain clinicians and researchers whose individual contributions were cornerstones of the process.
Probably the first to recognize the importance of mild upper respiratory illness in life-threatening events, if not apnea per se, were Jacob Werne and Irene Garrow.1 In 1947, as New York City medical examiners, these two were the first to challenge the then widely accepted mechanical suffocation theory of sudden infant death. Werne and Garrow cited a number of retrospective findings that led them to suspect alternative explanations for the phenomenon. They identified a number of infants who had been seemingly well, but expired in the presence of witnesses such that "there could be no possible allegation of smothering."1 Additionally, of the postmortem examinations in children younger than 1 year, those not demonstrating sufficient gross findings to explain the cause of death, instead frequently showed microscopic evidence of acute inflammation of the respiratory tracts. Consistent with these pathological findings were interviews with parents of SIDS cases that revealed a seeming disproportionate frequency of cases of upper respiratory illness just prior to death. Finally, they noted that the seasonal October-to-April increase in infant deaths previously certified as "accidental mechanical suffocation", closely paralleled the seasonal incidence of respiratory disease. Werne and Garrow did not propose a mechanism by which an upper respiratory illness might be responsible for infant deaths, but did opine that the relationship that they noted was most likely causal. Since then many other postmortem studies have identified the disproportionate presence of respiratory viruses in SIDS cases versus age-matched controls.2-4
Almost 20 years after Werne and Garrow's landmark paper, Stevens5 suggested that apnea was the proximate cause of death in sudden infant death associated with mild respiratory disease. Gathering personal and published observations, he pointed out that death by bacterial sepsis must take at least several hours to foment, during which time the infant should "be expected to become at least miserable and cry."5 Instead, suspecting a role for viral respiratory illness, he cited the more common occurrence is that the otherwise well infant "suddenly and quite unexpectedly…stops breathing, goes limp, and becomes blue, and there is no assurance that he will recover spontaneously." To support his assessment he provided detailed accounts of 4 typical cases each between 2-4 months of age, each with upper respiratory symptoms for 2-3 days, but who were otherwise considered to be "well up until the moment that they were observed, in the absence of choking or struggle, to stop breathing."5 In 3 of 4 cases the parents thought the infant had died, but were heartened when spontaneous recovery occurred. (In one case, artificial resuscitation was applied.) In all cases, at the time of hospital admission, the condition of all infants was "good relative to the grave state they were reported to have deteriorated to shortly before", however all died within 6 hours. While compelling, Steven's cases were not subjected to microbiologic study, the assumption that a respiratory virus was the cause remained only an assumption.
In 1967, two years after the publication of Steven's cases, P.S. Gardner and colleagues in Newcastle upon Tyne, England, noted that as a consequence of the advent of antibiotics there was a 50-fold decrease in the number of respiratory deaths in the 1-to-4-year-old group but a more moderate sixfold decrease in deaths in the younger-than-1-year age group.6 At the time of the report, a stunning 87% of the deaths from respiratory disease in children now were occurring during the first year of life. Gardner considered the 22 most recent deaths in Newcastle of children who were hospitalized for respiratory illness. Some of these children died from fulminant bacterial sepsis, or severe focal bacterial disease (e.g., lung abscesses, severe pneumonia, or epiglottitis). However, 11 of the 22 cases had either bronchiolitis or a mild respiratory tract infection. Nine of these 11 died suddenly "becoming pale, hypotonic with shallow irregular or gasping respirations, but without cardiac arrest."6 Four of these 9 who died suddenly, and in a total of 9 of the 22, RSV was isolated — for the first time implicating this virus in apneic deaths.
In the 1970's Steinschneider working with polysomnography cemented the understanding that prolonged apnea occurring in conjunction with upper respiratory tract infection could be the cause of some SIDS cases.7 While his initial 1972 article is tainted by the later discovery that 2 of the infants studied were ultimately the victims of infanticide,8,9 the study stands as early implication of respiratory viruses in the production of apnea, and prolonged apnea in sudden infant death. Later sleep studies that he performed on larger groups of infants confirmed the presence of apnea in infants during upper respiratory illnesses and further characterized the properties of the phenomenon: that it resolved when illness-free, and that it ceased to occur as the infants grew older.10,11
The mid 20th century was a time of explosive growth in virology such that isolation techniques that were unknown to Werne and Garrow were routine by the time Bruhn first systematically addressed the phenomenon of respiratory virus-associated apnea in 1977.12-14 Based upon the observation by local physicians that RSV seemed to cause apnea, Bruhn reviewed the clinical courses of infants with viral isolates from the microbiology laboratory in several Colorado hospitals. By comparing features of apneic infants with controls, he established risk factors for respiratory virus-associated apnea. Bruhn considered not only RSV, but other respiratory viruses as well. Since Bruhn's report, the number of respiratory viruses that have been implicated in the causation of apnea has grown. (See Table 1.)
| Table 1. Viruses Thought to Cause Apnea | ||
|
Respiratory Syncytial Virus
While a number of viruses have been documented to cause apnea in infants, RSV is the most important and best studied of these. The history of the discovery of RSV is interesting and serendipitous. Unknown before 1956, the virus was first isolated from a group of chimps at Walter Reed Army Institute of Research.15 The chimps had arrived from a Florida handler apparently well, and contracted an upper respiratory illness from an unclear source. The unplanned infection was nevertheless rigorously studied. Morris and colleagues reported the symptoms related to the virus, its transmissibility by nasal inoculation to other chimps, and finally noted its ability to pass to humans as had accidentally occurred in the laboratory.15 Based upon the most obvious symptom, Morris referred to the virus as the "chimp coryza virus." A year later Channock and researchers recovered the same virus from infants and young children with bronchiolitis and pneumonia in the nearby city of Baltimore.16 Based upon the microscopic appearance wrought by the virus on cells in culture, Channock renamed the agent respiratory syncytial virus. At that time there were three other known viruses that caused the formation of syncytia in tissue culture, one of them was also a respiratory virus; nevertheless, the term respiratory syncytial virus (RSV) has remained.
In 1960, additional reports from more disparate parts of the country were published, adding croup to the list of illnesses for which this virus could be responsible.17,18 Since then the list of illnesses caused by RSV has continued to grow. (See Table 2.)
| Table 2. Illnesses Attributable to RSV | ||||
|
||||
RSV is currently understood to be the single most important cause of respiratory illness in infants, infecting half of all infants during the first year of life.19 It is the most common etiologic agent of bronchiolitis19 and results in more than 160,000 hospitalizations of infants and young children annually in the United States.20 RSV is an enveloped, single-stranded RNA virus in the family Paramyxoviridae.19 The envelope contains surface proteins F (fusion) and G (glycoprotein) that are involved in infectivity and in immunogenicity. The G protein determines the RSV subtype (A and B), and, important to this paper, plays a role in the pathogenesis of apnea.21
Pathophysiology
RSV is passed through respiratory droplet exposure to nasal and ocular — and to a lesser extent oral — mucosal surfaces, sometimes with fomite participation in the chain of infection.22 After 4 to 5 days of incubation, the virus spreads through the respiratory epithelia of the nasopharynx by cell-to-cell and interstitial spread.23,24 Upper and lower respiratory tract symptoms result from both the direct cytopathic effects of the virus and the host inflammatory response, especially in terms of the cytotoxic T-cell effects.23,24
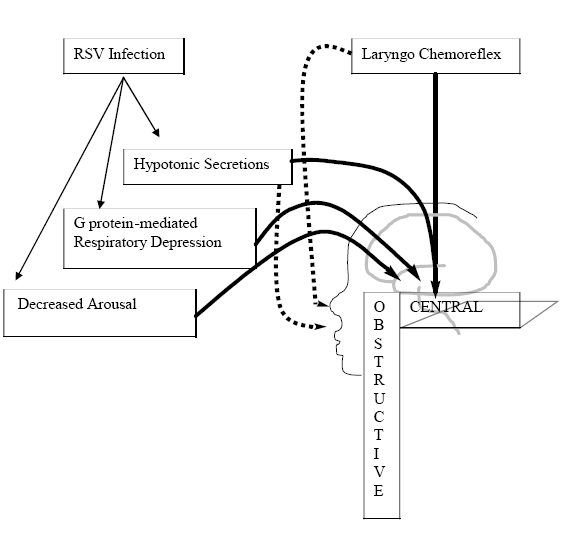
The mechanism of RSV-associated apnea is more complex and until recently, much more poorly understood. Anas reported that all prolonged apneic events were central.25 At first pass, isolated central apnea seems consistent with various other investigators' reports of cases wherein apnea occurred without the respiratory symptoms that would seem necessary to explain obstructive apnea.6,14 However, Pickens and researchers, based upon poly-somnography of two RSV-infected infants, reported finding mixed apnea, having both obstructive and central events.26 While their polysomnographic data do support this, it must be understood that obstructive apnea may not be independent of central processes. In other words, obstructive apnea may exist or be more notable in the presence of diminished central nervous system (CNS) arousal mechanisms.27
Our current state of understanding of the pathophysiologic mechanisms potentially responsible for RSV-associated apnea are discussed below and then summarized. Some of the mechanisms are not currently thought to be major contributors, but are presented for a thorough discussion.
Hypoxia
Hypoxia is a CNS depressant and can impair normal arousal to airway obstruction.28 Hospitalized RSV-infected apneic infants had, on the average, lower mean oxygen saturation (85%) than those infants who were not apneic (90%).29 However, in this study it is not clear that the apnea did not cause the hypoxia rather than the hypoxia causing the apnea. Others have found an increased A-a gradient in apneic versus nonapneic RSV-infected infants.25 Nevertheless, polysomnography does not support hypoxia as a trigger for RSV-associated apnea.25
Diaphragmatic Fatigue
Diaphragmatic and respiratory muscle fatigue leading to respiratory failure and apnea are a potential consequence of any illness with an obstructive respiratory component. Apneic RSV-infected infants have lower blood pH and higher pCO2 levels than those who are not apneic.29 However, the repeated observation of apnea in infants who lacked wheezing or retractions suggests that obstruction leading to diaphragmatic fatigue is not the usual cause of RSV-associated apnea.14,25,29
Nevertheless, there exists a second possible cause of dia-phragmatic fatigue. Diaphragmatic flutter, consisting of rapid involuntary contractions of the diaphragm occurring in the range of 150 to 480 per minute, is an uncommonly reported phenomenon.30 Some have suggested, however, that it is an under-recognized cause of respiratory failure in neonates.31 Adams and colleagues reported 3 infants with RSV infection and diaphragmatic flutter who were hospitalized for risk of apnea.30 In the case of these three infants, the events were brief (< 5 minutes) and did not result in clinical deterioration. Adams reported that the diaphragmatic flutter was not detectable by physical examination. However, most, but not all, who have reported this entity describe a clinically apparent di-rhythmic respiratory pattern representing superimposed frequencies of normal respiration and flutter.31-36 This finding suggests that if diaphragmatic flutter were the usual cause of RSV-associated apnea it would be noticed more often.
Central Nervous System Infection
Respiratory viruses by definition have a tropism for respiratory tissue, however, RSV infection may involve the brain as well.37-39 Thus, the potential exists for apnea to be the direct result of an encephalitic process or secondarily from encephalitis-wrought seizures. In Bruhn's initial study of apnea,14 cerebrospinal fluid samples from 40 apneic infants were compared with age-matched controls. Bruhn found no difference in cell counts between these two groups, suggesting that encephalitis is not the cause of most cases of RSV-associated apnea. And, while seizures have been associated with RSV infection,37,38 the fact that RSV-associated apnea can be induced by upper airway stimulation26 and ended by external tactile stimulation,40 suggests that seizures are not the cause.
Airway Protective Reflexes
Laryngeal chemoreceptors are one of several types of nervous receptors housed in the respiratory tract.41 The main role of these receptors is to protect against aspiration by modulating airway constriction, swallowing, cough, and cessation of respiration.42 The cessation of respiration may be advantageous in terms of prevention of aspiration, but deleterious in terms of ventilation. This reflex has been implicated in the causation SIDS,43,44 apnea of prematurity, reflux-associated apnea, and respiratory virus-associated apnea.42 Additionally, two critical pieces of evidence have come through animal research. First, there are age-related changes in the receptor's sensitivity that are consistent with the predominance of RSV-associated apnea in the very young and premature infants.45 Second, in live animal models, RSV infection appears to increase the effect of the laryngochemoreflex, resulting in a greater decrease in respiratory rate 46 and longer time to recovery of normal breathing.47 The clinician should be aware that other factors, such as sleep position, as well as postnatal nicotine and alcohol exposure, are also known to exacerbate the effect of the laryngochemoreflex and could have additive effects in the setting of RSV infection.44
RSV Functional Neurotoxins
Encephalopathy associated with RSV infection, in the absence of cerebrospinal fluid abnormalities, suggests the effect of neurotoxins.37 While less than 2% of those patients hospitalized with RSV infection have frank encephalopathy,37 lethargy remains a common symptom of RSV infection and viral respiratory disease in infants.38,48,49 It has been suggested that poor arousal may lead to delayed recovery from laryngochemoreflex apnea.50 This process has been demonstrated in RSV-infected lambs wherein the sleep state exacerbated the apneic periods.47
It has been hypothesized that inflammatory mediators such as some of the so-called somnogenic cytokines (IL-1,IL-2,Il-6, TNF- a, and IFN)44 play a role in SIDS. These cytokines could play a role in the lethargy produced by RSV. With regards to IL-1 a, TNF-a, and IL-6, however, there is no association between elevated levels and apnea in RSV-infected infants.51
Recently, Tripp and associates made important strides in our understanding of the role played by functional neurotoxins in RSV-associated apnea.21 A small portion of the G protein found on the surface envelope of the RSV has the ability to functionally mimic the chemokine fractalkine. Chemokines are proteins that have traditionally been described as being involved in leukocyte migration and activation.52 However, more recently it has been shown that neurons and supporting cells have receptors for chemokines as well.53,54 Tripp demonstrated that the RSV G protein, in otherwise uninfected mice, could cause a decrease in respiratory rates by inducing substance P release, a process normally modulated by fractalkine.21
Summary of Pathophysiology
Probably a number of pathophysiologic contributors to RSV-associated apnea exist, the most important being a triggered laryngochemoreceptor reflex in the setting of depressed respiratory drive mediated by functional neurotoxins. The clinician should additionally be aware that there are coincident risk factors for clinically important untoward effects on arousal and the laryngochemoreflex. These include the prone sleep position55,56 and postnatal nicotine57 and alcohol58 exposure. A summary schematic diagram relates the proposed contributions of various pathophysiologic mechanisms (Figure 1).
| Figure 1. Pathophysiologic Contributors to RSV-associated Apnea |
|
Diagnosis
The diagnosis of respiratory virus-associated apnea consists of documentation or suspicion of one or more apneic events in the clinical setting of a respiratory infection. In the absence of a formalized definition of apnea that is specific to this setting, we borrow from previous definitions: respiratory pauses of 15 to 20 seconds, or shorter pauses that result in desaturation or bradycardia.59,60
While respiratory infection is a clinical diagnosis, efforts should be made to identify the offending organism. Failure to identify a respiratory virus known to cause apnea (See Table 1) by rapid testing in the ED should raise suspicion of an alternative diagnosis and prompt additional evaluation. Alternative diagnoses that could result in apnea in an infant include sepsis, meningitis, encephalitis, seizures, and gastroesophageal reflux. The well appearance of many infants between apneic episodes might help to eliminate the need for more invasive evaluation; however, this possibility has not been submitted to scientific scrutiny.
Laboratory methods used to diagnose respiratory viruses include tissue culture, rapid direct specimen antigen tests (membrane enzyme immunoassays [EIAs], and both direct and indirect immunofluorescent assays [DFAs], and monospecific and multiplex polymerase chain reaction [PCR]).61-63 Specimens are obtained by either nasopharyngeal (NP) aspirate or NP swabbing. For RSV, NP aspirate appears to provide higher sensitivity.64,65
Isolation of respiratory viruses by viral culture is considered the gold standard for laboratory diagnosis and usually is performed in combination with rapid direct specimen antigen tests (a turnaround time of about 12 hours for rapid direct specimen antigen tests can be expected). Direct specimen antigen tests demonstrate varying sensitivities, therefore at many institutions a backup culture is performed on negative specimens.63 Tissue culture results are usually not available for 2 to 10 days and, therefore, have little effect on patient care.62,63 Due to the long turnaround time of culture and varying sensitivities of rapid direct specimen antigen tests, PCR is an alternative testing method. PCR is a rapid, sensitive, and specific method for the detection of seven of the most common respiratory viruses in children. PCR can detect parainfluenza virus types 1, 2, and 3, RSV types A and B, and influenza virus types A and B. Results are available in about 6 hours. The sensitivity for RSV and influenza A is 98.6% and 95.8%, respectively.63
Therapy
The treatment of infants suffering from respiratory virus-induced apnea depends upon the severity of symptoms and the clinical setting. For those who are most severe, bag-mask ventilation with supplemental oxygen should be employed while preparations are made for endotracheal intubation and mechanical ventilation. Because, in almost all cases, apnea continues for only a few days,14,25 tracheostomy is almost never indicated.
In those patients who are less severe, and in cases wherein the clinical setting allows immediate availability of expert airway management, close monitoring and intermittent airway management may be an option. In some cases, tactile stimulation effectively — but temporarily — improves respiratory effort allowing more relaxed decision making.44 However, the clinician must be aware that infants with a history of a life-threatening apneic event may appear well after the stimulation that is part of ED intake and triage, and, yet, be a great risk of prolonged apneic events after initial assessment.
A few novel approaches have made their way into the literature. The most important of these is the use of methylxanthines. Debuse first reported a case of apparent benefit from an oral theophylline load effective several hours after administration.66 Later, intravenous theophylline was reported to rapidly end respiratory virus-induced apnea in 2 infants.67 Most recently, Tobias retrospectively compared frequency of RSV-associated apnea before and after intravenous caffeine administration in 7 infants, noting a statistically significant and clinically marked decrease after caffeine administration.40 McNamara reported a case wherein nasal continuous positive airway pressure (NCPAP) was used to effectively overcome the obstructive component of respiratory virus-induced apnea.68 Central apnea continued in this infant, but while on the NCPAP, there was no desaturation below 91%. Rockney reported the use of ribavirin in 2 infants with RSV-associated apnea; its effectiveness was not clear.69
Untried Therapies
There are a number of potential therapies whose effectiveness has not been demonstrated outside of animal models, or whose effectiveness is entirely theoretical.
Palivizumab. Palivizumab is a monoclonal antibody aimed against the RSV glycoprotein F.70 It is intended for passive immunoprophylaxis of infants who were premature, as well as young children with chronic lung disease or hemodynamically significant congenital heart disease. For these select groups, immunoprophylaxis has been shown to decrease the risk of hospitalization with RSV infection.70 The effect of passive immunoprophylaxis on the risk of apnea in human infants has not been studied. However, investigators studying weanling rats found that palivizumab given prior to inoculation of RSV effectively reduced RSV-associated apnea.71 This effect was less marked but still present even when the palivizumab was administered 72 hours after inoculation, opening the door to the possible use of this agent for treatment of RSV-associated apnea. While unstudied, in theory RSV intravenous immunoglobin (IVIG) might be more effective because of the presence of anti-glycoprotein G antibodies.72
Beta-Adrenergic Agonists. Agents that preferentially stimulate beta type two receptors could play a role in the future treatment of RSV-associated apnea. The laryngochemoreflex-related apnea is attenuated by terbutaline in newborn lambs.73 The value that a beta type two agonist might have in the treatment of RSV-associated apnea in human infants is not known. Nevertheless, in one retrospective study of 10 RSV-infected infants with apnea, the one infant with the least apnea was also the only one who had received therapy with a beta two agonist, (presumably used for purposes of bronchodilation).51 While the evidence is weak, the safety record of these agents and frequent use during RSV infection for bronchiolitis serve to raise interest in these agents. The effect of mixed adrenergic agents (e.g., epinephrine) on respiratory virus-associated apnea or on the laryngochemoreflex is not known.
Other Agents. Diphenhydramine decreases laryngochemoreflex-induced apnea in neonatal piglets when given intravenously or in nebulized form.74,75 Topical (nebulized), but not intravenous, lidocaine seems to temporarily abolish the laryngochemoreflex in piglets.76,77 Other currently available agents that could theoretically benefit infants with RSV-associated apnea might include CNS stimulants and muco-suppressive agents, however, these have not been studied. The value in respiratory virus-associated apnea of other agents more commonly used for treatment of bronchiolitis, such as heliox or glucocorticoids, is not known.
The At-Risk Infant
The presence of apnea historically or on physical examination is a major risk factor for additional episodes of apnea.25,29 However, it has long been understood that apnea and subsequent death can occur in infants with upper respiratory symptoms but who are otherwise well and have no history of apnea on presentation.5,6,14 This has been a major concern to health care providers managing infants with symptoms of upper respiratory illness, especially if there is a concern that the etiologic agent might be RSV. Thus, decisions regarding disposition may be difficult for any young infant with cough and nasal congestion whose temperature or lower airways disease does not dictate hospitalization. There are more than 20 entries discussing the issue of risk of apnea in at-risk infants on the pediatric emergency medicine listserv without clear concensus on the best approach.78 A number of studies have sought to determine risk factors for apnea in RSV-infected infants.
As mentioned previously, Bruhn and colleagues reviewed the medical charts of 274 infants younger than 6 months in the Denver area from whom viral isolation at area laboratories had yielded RSV.14 Apnea was documented to have occurred in 56 patients (20%) of the group that, for the most part, was represented by infants ill enough to be hospitalized. There were 4 deaths in the group, but none of the deaths were thought to have been a direct result of the apnea. A number of key risk factors were identified, some of which have continued to be born out in more contemporary research. Chief among these are the associations with prematurity and young chronological age.
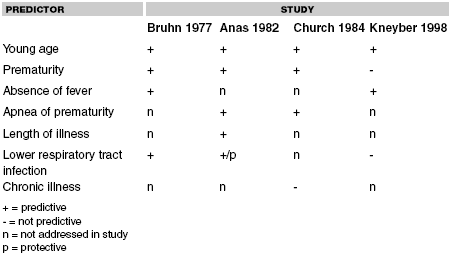
Following Bruhn's study, several other descriptive studies were undertaken to better characterize the nature of RSV-associated apnea: Anas et al25 in 1982, Church et al79 in 1984, and Kneyber et al29 in 1998. For each of these, the study group was retrospectively identified from laboratory specimens indicating the presence of RSV, while clinical data were obtained from chart review. It is important to understand that the Anas paper represented a small group of 32 children younger than 18 months, only 5 of which suffered apnea. Many from this group were later incorporated into Church's larger group of 261 infants seen at the same institution but over a longer time period. Thus, the two studies do not necessarily represent independent information but are presented separately because some different independent variables are evaluated from one study to the next. Kneyber's study is of particular importance. Because it was conducted more recently, it incorporated more modern statistical analysis. This included multivariate linear and logistic regression that allowed the ability to determine the independent contribution of otherwise confounding variables. Current knowledge about which variables might predict apnea is addressed below.
Predictors of Apnea
Chronological Age. In Bruhn's original study, 43% of all of the apneic infants were younger than 1 month, 75% were younger than 2 months, and 91% were younger than 3 months.14 Similarly, Church79 and Kneyber29 both found that infants with RSV and apnea averaged roughly 2 months of age, and were on the average a full month younger than RSV-infected infants who did not suffer apnea.
While younger chronologic age generally appears to be a consistent risk factor for RSV-associated apnea, one prospective study limited to neonates found that those younger than 3 weeks were less likely to suffer apnea than those older than 3 weeks.80 However, this finding could be reflective of the fact that those infants older than 3 weeks in the neonatal intensive care unit might be more likely to have been more premature at birth than those who were on average chronologically younger.
Gestational Age. In Bruhn's study, 57% of the apneic infants had a history of prematurity versus only 20% of the nonapneic group.14 Church found similar percentages in each group, and additionally reported the average apneic infant to have been born at 36 weeks versus 40 weeks for the average nonapneic RSV-infected infant.79 Kneyber did not find prematurity to be an independent predictor of apnea.29
While less compelling, in one descriptive study of 40 neonates with viral pneumonia, investigators noted that apnea occurred more frequently in premature versus full-term neonates (56% vs 23% at any time) (44% vs 13% during examination).48 However, these differences did not reach statistical significance in this small study.
Apnea of Prematurity. Anas found that a history of apnea of prematurity was present in 4 of 5 apneic infants versus 0 of 27 nonapenic infants.25 Church explored this further, attempting to control for the confounding effects on gestational age by comparing only those younger than 32 weeks gestation.79 Using this stratification, Church found that 90% of those in the apneic group had a history of apnea of prematurity versus 45% of those who did not have apnea related to their current illness, suggesting that this is an independent predictor of respiratory virus-associated apnea. Both the Bruhn14 and Kneyber29 studies mentioned a small number of infants who had a history of apnea of prematurity, however, no comparative analyses was made.
Underlying Disorders. While serious underlying disease increases the risk of the severity of RSV-related disease,81-83 limited evidence suggests that it does not appear to increase the risk of apnea.79
Fever. The presence of fever does not increase, but rather decreases the likelihood of apnea. Bruhn's study suggested that those patients without apnea were twice as likely to be febrile on presentation.14 Kneyber reported the effect of temperature in different terms: those with apnea had an average temperature of 37.3°C versus those without apnea had an average temperature of 38.0°C; while the difference seems small it was highly statistically significant.29 It is not known whether the lack of fever is truly an independent predictor of apnea or if it is perhaps related to other predictors such as prematurity, chronological age, or length of illness.
Length of Illness. Anas reported that those patients with apnea presented with an average of 2 days of respiratory symptoms, versus almost 4 days for those without apnea.25 While others have not looked at length of illness in a systematic fashion, additional information can be inferred that suggests that this might be an important predictor. Bruhn reported that 2 of the apneic infants in his study had no respiratory signs on presentation but only developed these later, suggesting that apnea may be an early symptom of RSV infection.14 He additionally noted the presence of otitis media, potentially a late superinfection, was more common in those without apnea.
Lower respiratory symptoms usually occur later, after upper respiratory symptoms in RSV infection.23 Anas reported that the presence of wheezing, a marker of lower respiratory tract infection, was less common in those patients with apnea than those without.25 Other researchers, however, have not found this difference.14,29
Lower Respiratory Tract Illness. Predictability of the presence or absence of various findings attributable to lower respiratory tract illness has been conflicting. Anas found infiltrates on chest x-ray predictive, while the presence of wheezing was somewhat protective against apnea.25 Kneyber found atelectasis on chest x-ray to be predictive of apnea.29 Bruhn found that lower respiratory illness was neither predictive nor protective of apnea.14
Clinical Approach
From the Bruhn14, Anas,25 Church,79 and Kneyber29 studies, we can identify that infants at greatest risk of respiratory virus-associated apnea are those who are younger, have a history of prematurity, a history of apnea of prematurity, and those whose duration of illness is shorter. (See Table 3.) From pathophysiologic studies of the laryngochemoreflex, we also would have increased concerns about those infants who are nicotine or alcohol exposed,57,58 have a tendency to sleep prone,55,56,83 and do not use a pacifier.84
| Table 3. Evidence for Predictors of Apnea in Young Infants |
|
It is important that these predictors not be construed as a clinical algorithm that can predict which infants will or will not have apnea following a clinical interface. Rather these risk factors as 'tests' have unknown sensitivity or specificity. There is no objectively tested clinical prediction rule that can identify which infants can be safely discharged home, yet monitored admission is not feasible in every case of upper respiratory infection in those younger than 1 year. Instead a balance must be drawn on a case-by-case basis between the risk of clinically important apnea, and the economic and noneconomic costs of hospitalization, including the risks of nosocomial infection and iatrogenic illness.
One option to help gauge the risk of apnea is to perform rapid tests for the presence of RSV antigen in nasal secretions. However, rapid tests for RSV antigen may have sensitivities ranging from as low as 61% to as high as 97% depending upon the technique.64,65 Further, other respiratory viruses for which rapid tests may not be available also can cause apnea. During the course of an ED visit, the presence of apnea following nasal saline lavage or during a cardiorespiratory and oxygen saturation monitored period (ideally including a feeding and sleep period) can help identify infants not initially considered at high risk of apnea. Additionally, some have supported the practice of admitting all infants younger than 2 months identified to be infected by RSV.77 However, this practice has not been evaluated.
Recently Willwerth and colleagues retrospectively applied a series of risk criteria to a set of infants younger than 6 months hospitalized for respiratory disease.85 Infants were considered high risk if they fit any of the following criteria: 1) full term and younger than 1 month, 2) premature (< 37weeks) and postgestational age less than 48 weeks, or 3) presenting with apnea. These criteria identified all infants who had subsequent apnea during the hospitalization. However, it is not known if these criteria can be used to determine safe disposition from the ED of young infants who would not otherwise be hospitalized for their respiratory disease.
Home Monitoring
The usefulness of home monitoring for the specific concern of respiratory virus-associated apnea has not been evaluated. In the 1970's, Steinschneider originally espoused liberal use of the home monitor for at risk infants.7,11,86 This was more or less supported by the 1986 National Institutes of Health Consensus Development Conference statement that home apnea monitoring was appropriate for certain infants deemed to be at high risk of sudden infant death, including some of those who had suffered apparent life threatening events.87 The more recent 2003 American Academy of Pediatrics (AAP) policy statement on "Apnea, Sudden Infant Death Syndrome, and Home Monitoring" was less supportive of home monitoring in general; however, it acknowledged a role for home monitoring in certain cases, including infants presenting with apparent life-threatening events, for the purpose of detecting apnea.88
Concerns raised by those who do not favor the use of home monitors include the paucity of evidence that the alarms can effectively result in prevented deaths, as well as economic considerations.88 However, there is room for alternative interpretation of some of the most important home monitoring literature.89-91
Those critically reviewing this literature will find that the ability to draw unambiguous inferences is limited. Infantile apnea resulting in death, as a thankfully rare dependent variable, necessarily results in either underpowered studies that bias toward the null, or the use of surrogate variables that do not reflect the study question as accurately.
Additionally, there is inherent systematic bias that exists against finding a benefit of monitoring for apnea in the prevention of infantile death. That is, monitoring that demonstrates prolonged apnea by design might decrease the probability of progressing to death, while deaths might regularly occur in the apparent absence of prolonged apnea in unmonitored infants because the apnea is not suspected or detected. Ergo, finding no difference in the risk of death of infants monitored for apnea and those who are unmonitored neither implies that apnea does not lead to infant death nor that monitoring is ineffective at reducing the risk of death.
While many descriptive studies have documented successful resuscitation by parents of events detected with home monitoring,91-93 these parents were trained in the resuscitation of infants and were armed with bag-mask-valve ventilators. Further, despite these precautions, death still occurred in some cases despite the presence of home monitoring.90,91 Therefore, hospitalization is required for infants suspected of having respiratory virus-associated apnea. Home monitoring should be reserved for at-risk infants who are not at high risk, have not demonstrated apnea, and whose parents are properly trained, equipped, and willing to provide resuscitation should the need arise.
Summary
Respiratory virus-associated apnea is defined as the presence of apnea in the setting of a viral respiratory illness, and in the absence of another identifiable acute cause of apnea. RSV is the most common cause of respiratory virus-associated apnea. In the case of RSV, apnea appears to be the result of untoward effects of airway protective mechanisms in the setting of diminished respiratory drive. The pathophysiology of respiratory virus-associated apnea is not determined for viruses other than RSV. Younger infants with recent onset of respiratory symptoms — especially if born prematurely and with a history of apnea of prematurity — are at greatest risk of respiratory virus-associated apnea. The well appearance of some infants between apneic events, especially after the stimulation that can be part of ED intake, can cloud recognition of this entity in the fast-paced ED setting. Unrecognized, respiratory virus-associated apnea may result in death. When appropriately recognized, and depending upon the severity, a number of therapeutic options can be considered, including intubation and mechanical ventilation. Particularly challenging to the clinician is the clinical conundrum of the young infant with a respiratory illness who is at risk of respiratory virus-associated apnea, but in whom no events have yet occurred.
References
1. Werne J, Garrow I. Sudden deaths of infants allegedly due to mechanical suffocation. J Pub Health 1947;37:675-687.
2. Williams AL, Uren EC, Bretherton L. Respiratory viruses and sudden infant death. Br Med J (Clin Res Ed) 1984 May 19;288(6429):1491-1493.
3. An SF, Gould S, Keeling JW, Fleming KA. Role of respiratory viral infection in SIDS: detection of viral nucleic acid by in situ hybridization. J Pathol 1993 Dec;171(4):271-278.
4. Bajanowski T, Wiegand P, Cecchi R, et al. Detection and significance of adenoviruses in cases of sudden infant death. Virchows Arch 1996 May;428(2):113-118.
5. Stevens LH. Sudden unexplained death in infancy. Observations on a natural adoption of the face down position. Am J Dis Child 1965 Sep;110:243-247.
6. Gardner P, Turk D, Aherne W, et al. Deaths associated with respiratory tract infection in childhood. BMJ 1967;4:316-320.
7. Steinschneider A. Prolonged apnea and the sudden infant death syndrome: clinical and laboratory observations. Pediatrics 1972 Oct; 50(4):646-654.
8. Hickey C, O'Brien J, Lightly T. SIDS researcher sidestepped critics medical records don't support Dr. Alfred Steinschneider's claim that Waneta Hoyt's babies suffered severe breathing problems in the hospital. The Post-Standard May 6,1996. Syracuse NY.
9. Pinholster G. SIDS paper triggers a murder charge. Science 1994 Apr 8;264(5156):197-198.
10. Steinschneider A. Nasopharyngitis and the sudden infant death syndrome. Pediatrics 1977 Oct;60(4):531-533.
11. Steinschneider A. Nasopharyngitis and prolonged sleep apnea. Pediatrics 1975 Dec;56(6):967-971.
12. Melnick J. Nomenclature and classification of viruses. In: Feigin R, Cherry J, (eds). Textbook of Pediatric Infectious Diseases. 4th edition. Philadelphia: W.B. Saunders;1998: pp.1600-1620.
13. Brenner S, Horne R. A negative staining method for high resolution electron microscopy of viruses. Biochim Biophys Acta 1959;34:103-110.
14. Bruhn FW, Mokrohisky ST, McIntosh K. Apnea associated with respiratory syncytial virus infection in young infants. J Pediatr 1977 Mar;90(3):382-386.
15. Blount R, Morris J, Savage R. Recovery of cytopathogenic agent from chimpanzees with coryza. Proc Soc Exp Biol Med 1956 Jul;92(3):544-549.
16. Chanock R, Roizman B, Myers R. Recovery from infants with respiratory illness of a virus related to chimpanzee coryza agent (CCA). I. Isolation, properties and characterization. Am J Hyg 1957 Nov;66(3): 281-290.
17. Beem M, Wright F, Hamre D, et al. Association of the chimp coryza agent with acute respiratory disease in children. NEJM 1960;263(11): 523-530.
18. Rowe D, Michaels R. Isolation of the respiratory syncytial virus from a patient with pneumonia. Pediatrics 1960 Oct;26:623-629.
19. Welliver RC. Respiratory syncytial virus and other respiratory viruses. Pediatr Infect Dis J 2003 Feb;22(2 Suppl):S6-10; disc S10-12.
20. Shay DK, Holman RC, Newman RD, et al. Bronchiolitis-associated hospitalizations among US children, 1980-1996. JAMA 1999 Oct 20;282(15):1440-1446.
21. Tripp RA, Dakhama A, Jones LP, et al. The G glycoprotein of respiratory syncytial virus depresses respiratory rates through the CX3C motif and substance P. J Virol 2003 Jun;77(11):6580-6584.
22. Hall CB. Respiratory syncytial virus: its transmission in the hospital environment. Yale J Biol Med 1982 May-Aug;55(3-4):219-223.
23. Jafri HS. Treatment of respiratory syncytial virus: antiviral therapies. Pediatr Infect Dis J 2003 Feb;22(2 Suppl):S89-92; disc S92-93.
24. DeVincenzo JP. Factors predicting childhood respiratory syncytial virus severity: what they indicate about pathogenesis. Pediatr Infect Dis J 2005 Nov;24(11 Suppl):S177-183
25. Anas N, Boettrich C, Hall CB, Brooks JG. The association of apnea and respiratory syncytial virus infection in infants. J Pediatr 1982 Jul;101(1):65-68.
26. Pickens DL, Schefft GL, Storch GA, Thach BT. Characterization of prolonged apneic episodes associated with respiratory syncytial virus infection. Pediatr Pulmonol 1989;6(3):195-201.
27. Berry RB, Gleeson K. Respiratory arousal from sleep: mechanisms and significance. Sleep 1997 Aug;20(8):654-675.
28. Hlavac MC, Catcheside PG, McDonald R, et al. Hypoxia impairs the arousal response to external resistive loading and airway occlusion during sleep. Sleep 2006 May 1;29(5):624-631.
29. Kneyber MC, Brandenburg AH, de Groot R, et al. Risk factors for respiratory syncytial virus associated apnoea. Eur J Pediatr 1998 Apr;157(4):331-335.
30. Adams JA, Zabaleta IA, Sackner MA. Diaphragmatic flutter in three babies with bronchopulmonary dysplasia and respiratory syncytial virus bronchiolitis. Pediatr Pulmonol 1995 May;19(5):312-316.
31. Katz ES, Gauda E, Crawford T, et al. Respiratory flutter syndrome: an underrecognized cause of respiratory failure in neonates. Am J Respir Crit Care Med 2001 Oct 1;164(7):1161-1164.
32. Cvietusa PJ, Nimmagadda SR, Wood R, Liu AH. Diaphragmatic flutter presenting as inspiratory stridor. Chest 1995 Mar;107(3):872-875.
33. Stadler HE. Paroxysmal diaphragmatic flutter in a young infant. J Indiana State Med Assoc 1976 Mar;69(3):151.
34. Riordan LL, Eavey RD, Strieder DJ. Neonatal diaphragmatic flutter. Pediatr Pulmonol 1990;8(3):209-211.
35. Harrison P, Onorato DJ. Diaphragmatic flutter emulating recalcitrant asthma. South Med J 1997 Feb;90(2):234-236.
36. Corbett CL. Diaphragmatic flutter. Postgrad Med J 1977 Jul;53(621): 399-402.
37. Ng YT, Cox C, Atkins J, Butler IJ. Encephalopathy associated with respiratory syncytial virus bronchiolitis. J Child Neurol 2001 Feb;16(2):105-108.
38. Eisenhut M. Extrapulmonary manifestations of severe respiratory syncytial virus infection -a systematic review. Crit Care 2006; 10(4):R107.
39. Sweetman LL, Ng YT, Butler IJ, Bodensteiner JB. Neurologic complications associated with respiratory syncytial virus. Pediatr Neurol 2005 May;32(5):307-310.
40. Tobias JD. Caffeine in the treatment of apnea associated with respiratory syncytial virus infection in neonates and infants. South Med J 2000 Mar;93(3):294-296.
41. Widdicombe JG. Pulmonary and respiratory tract receptors. J Exp Biol 1982 Oct;100:41-57.
42. Thach BT. Reflux associated apnea in infants: evidence for a laryngeal chemoreflex. Am J Med 1997 Nov 24;103(5A):120S-124S.
43. Downing SE, Lee JC. Laryngeal chemosensitivity: a possible mechanism for sudden infant death. Pediatrics 1975 May;55(5):640-649.
44. Raza MW, Blackwell CC. Sudden infant death syndrome, virus infections and cytokines. FEMS Immunol Med Microbiol 1999 Aug 1:25(1-2):85-96.
45. Blum DJ, McCaffrey TV. Age-related responses of laryngeal airway resistance to peripheral and central chemoreceptor stimulation. Ann Otol Rhinol Laryngol 1984 Sep-Oct;93(5 Pt 1):488-493.
46. Lindgren C, Jing L, Graham B, Grogaard J, Sundell H. Respiratory syncytial virus infection reinforces reflex apnea in young lambs. Pediatr Res 1992 Apr;31(4 Pt 1):381-385.
47. Lindgren C, Lin J, Graham BS, et al. Respiratory syncytial virus infection enhances the response to laryngeal chemostimulation and inhibits arousal from sleep in young lambs. Acta Paediatr 1996 Jul;85(7):789-797.
48. Abzug MJ, Beam AC, Gyorkos EA, Levin MJ. Viral pneumonia in the first month of life. Pediatr Infect Dis J 1990 Dec;9(12):881-885.
49. Baker KA, Ryan ME. RSV infection in infants and young children. What's new in diagnosis,treatment, and prevention? Postgrad Med 1999 Dec;106(7):97-9, 103-104, 107-108 passim.
50. Marchal F, Corke BC, Sundell H. Reflex apnea from laryngeal chemo-stimulation in the sleeping premature newborn lamb. Pediatr Res 1982 Aug;16(8):621-627.
51. Lindgren C, Grogaard J. Reflex apnoea response and inflammatory mediators in infants with respiratory tract infection. Acta Paediatr 1996 Jul;85(7):798-803.
52. Ali S, O'boyle G, Mellor P, Kirby JA. An apparent paradox: Chemokine receptor agonists can be used for anti-inflammatory therapy. Mol Immunol 2007;Mar;44(7):1477-1482. Epub 2006 Sep 26.
53. Bajetto A, Bonavia R, Barbero S, et al. Characterization of chemokines and their receptors in the central nervous system: physiopathological implications. J Neurochem 2002 Sep;82(6):1311-1329.
54. Bajetto A, Bonavia R, Barbero S, Florio T, Schettini G. Chemokines and their receptors in the central nervous system. Front Neuroendocrinol 2001 Jul;22(3):147-184. Mol Immunol 2006 Sep 23; [Epub ahead of print]
55. Jeffery HE, Megevand A, Page H. Why the prone position is a risk factor for sudden infant death syndrome. Pediatrics 1999 Aug;104(2 Pt 1):263-269.
56. Horne RS, Ferens D, Watts AM, et al. The prone sleeping position impairs arousability in term infants. J Pediatr 2001;138(6):811-816.
57. Sundell HW, Karmo H, Milerad J. Impaired cardiorespiratory recovery after laryngeal stimulation in nicotine-exposed young lambs. Pediatr Res 2003 Jan;53(1):104-112.
58. Grogaard J, Van den Abbeele A, Sundell H. Effect of alcohol on apnea reflexes in young lambs. J Appl Physiol 1985;59(2):420-425.
59. Committee on Fetus and Newborn. American Academy of Pediatrics. Apnea, sudden infant death syndrome, and home monitoring. Pediatrics 2003 Apr;111(4 Pt 1):914-917.
60. McNamara DG, Nixon GM, Anderson BJ. Methylxanthines for the treatment of apnea associated with bronchiolitis and anesthesia. Paediatr Anaesth 2004 Jul;14(7):541-50.
61. Templeton KE, Scheltinga SA, Beersma MF. Rapid and sensitive method using multiplex real-time PCR for diagnosis of infections by influenza A and influenza B viruses, respiratory syncytial virus, and parainfluenza Viruses 1, 2, 3, and 4. J Clin Microbiol 2004;42:1564-1569.
62. Shetty AK, Treynor E, Hill DW. Comparison of conventional viral cultures with direct fluorescent antibody stains for diagnosis of community-acquired respiratory virus infections in hospitalized children. Pediatr Infect Dis J 2003:22:789-794.
63. Kehl SC, Henrickson KJ, Hua W. Evaluation of the hexaplex assay for detection of respiratory viruses in children. J Clin Microbiol 2001;39:1696-1701.
64. Ahluwalia G, Embree J, McNicol P, et al. Comparison of nasopharyngeal aspirate and nasopharyngeal swab specimens for respiratory syncytial virus diagnosis by cell culture, indirect immunofluorescence assay, and enzyme-linked immunosorbent assay. J Clin Microbiol 1987 May;25(5):763-767.
65. Macfarlane P, Denham J, Assous J, Hughes C. RSV testing in bronchiolitis: Which nasal sampling method is best? Arch Dis Child 2005 Jun;90(6):634-635.
66. DeBuse P, Cartwright D. Respiratory syncytial virus with apnoea treated with theophylline. Med J Aust 1979 Sep 22;2(6):307-308.
67. Johnston DM, Kuzemko JA. Virus-induced apnoea and theophylline. Lancet 1992 Nov 28;340(8831):1352.
68. McNamara F, Sullivan CE. Nasal CPAP treatment in an infant with respiratory syncytial virus-associated apnea. Pediatr Pulmonol 1997 Sep;24(3):218-221.
69. Rockney R. Ribavirin and respiratory syncytial virus—associated apnea. Am J Dis Child 1988 Sep;142(9):913.
70. American Academy of Pediatrics Committee on Infectious Diseases and Committee on Fetus and Newborn. Revised indications for the use of palivizumab and respiratory syncytial virus immune globulin intravenous for the prevention of respiratory syncytial virus infections. Pediatrics 2003 Dec;112(6 Pt 1):1442-1446.
71. Sabogal C, Auais A, Napchan G, et al. Effect of respiratory syncytial virus on apnea in weanling rats. Pediatr Res 2005 Jun;57(6):819-825. Epub 2005 Mar 17.
72. Hemming VG, Prince GA, Groothuis JR, Siber GR. Hyperimmune globulins in prevention and treatment of respiratory syncytial virus infections. Clin Microbiol Rev 1995;8:22-33.
73. Grogaard J, Sundell H. Effect of beta-adrenergic agonists on apnea reflexes in newborn lambs. Pediatr Res 1983 Mar;17(3):213-219.
74. Downs DH, Johnson K, Goding GS Jr. The effect of antihistamines on the laryngeal chemoreflex. Laryngoscope 1995 Aug;105(8 Pt 1):857-861.
75. Boyer HC, Downs DH, Goding GS Jr, Pernell KJ. Effect of topical diphenhydramine on the laryngeal chemoreflex. Arch Otolaryngol Head Neck Surg 1996 Oct;122(10):1112-1116.
76. McCulloch TM, Flint PW, Richardson MA, Bishop MJ. Lidocaine effects on the laryngeal chemoreflex, mechanoreflex, and afferent electrical stimulation reflex. Ann Otol Rhinol Laryngol 1992 Jul; 101(7):583-589.
77. Lee JC, Stoll BJ, Downing SE. Properties of the laryngeal chemoreflex in neonatal piglets. Am J Physiol 1977 Jul;233(1):R30-36.
78. Pediatric emergency medicine listserv (PED-EM-L ) accessed 10/09/06. Available: http://www.brown.edu/Administration/Emergency_Medicine/ped-em-l.html
79. Church NR, Anas NG, Hall CB, Brooks JG. Respiratory syncytial virus-related apnea in infants. Demographics and outcome. Am J Dis Child 1984 Mar;138(3):247-250.
80. Hall CB, Kopelman AE, Douglas RG Jr, et al. Neonatal respiratory syncytial virus infection. N Engl J Med 1979 Feb 22;300(8):393-396.
81. Hall CB, Powell KR, MacDonald NE, Respiratory syncytial virus infection in children with compromised immune function. N Engl J Med 1986; 315:77-81.
82. Groothuis JR, Gutierrez KM, Lauer BA Respiratory syncytial virus infection in children with bronchopulmonary dysplasia. Pediatrics 1988; 82:199-203.
83. MacDonald NE, Hall CB, Suffin SC, et al. Respiratory syncytial viral infection in infants with congenital heart disease. N Engl J Med 1982 Aug 12;307(7):397-400.
84. Lindgren C. Respiratory control during upper airway infection mechanism for prolonged reflex apnoea and sudden infant death with special reference to infant sleep position. FEMS Immunol Med Microbiol 1999 Aug 1;25(1-2):97-102.
85. Willwerth BM, Harper MB, Greenes DS. Identifying hospitalized infants who have bronchiolitis and are at high risk for apnea. Ann Emerg Med 2006 Oct;48(4):441-447.
86. Steinschneider A. A re-examination of "the apnea monitor business." Pediatrics 1976 Jul;58(1):1-5.
87. National Institutes of Health Consensus Development Conference on Infantile Apnea and Home Monitoring, Sept 29 to Oct 1, 1986. Pediatrics 1987 Feb;79(2):292-299.
88. Committee on Fetus and Newborn. American Academy of Pediatrics. Apnea, sudden infant death syndrome, and home monitoring. Pediatrics 2003 Apr;111(4 Pt 1):914-917.
89. Ramanathan R, Corwin MJ, Hunt CE, et al. Collaborative Home Infant Monitoring Evaluation (CHIME) Study Group. Cardiorespiratory events recorded on home monitors: Comparison of healthy infants with those at increased risk for SIDS. JAMA 2001 May 2;285(17):2199-2207.
90. Ward SL, Keens TG, Chan LS, et al. Sudden infant death syndrome in infants evaluated by apnea programs in California. Pediatrics 1986 Apr;77(4):451-458.
91. Duffty P, Bryan MH. Home apnea monitoring in 'near-miss' sudden infant death syndrome (SIDS) and in siblings of SIDS victims. Pediatrics 1982 Jul;70(1):69-74.
92. MacKay M, Abreu e Silva FA, MacFadyen UM, et al. Home monitoring for central apnoea. Arch Dis Child 1984 Feb;59(2):136-142
93. Tudehope DI, Cleghorn G. Home monitoring for infants at risk of the sudden infant death syndrome. Aust Paediatr J 1984 May;20(2):137-140.
Apnea is a frightening event for the parent and a challenging diagnostic evaluation for the emergency department (ED) physician.Subscribe Now for Access
You have reached your article limit for the month. We hope you found our articles both enjoyable and insightful. For information on new subscriptions, product trials, alternative billing arrangements or group and site discounts please call 800-688-2421. We look forward to having you as a long-term member of the Relias Media community.